Indeterminate Biliary Stricture – Important Concepts You Need to Know
The clinical presentation of biliary strictures is broad, ranging from incidental imaging findings and laboratory results to symptoms of jaundice, abdominal pain, pruritus, and cholangitis. Therefore, a detailed clinical history can provide important information about its etiology.
Biliary stricture is considered a narrowing of the biliary tree that can be caused by a myriad of etiologies, some benign, others life-threatening.
- There are three classes of biliary strictures: benign, malignant, and indeterminate.
- Unfortunately, only a minority of them (15% to 24%) are benign.
Differentiation between benign and malignant requires a complex diagnostic evaluation, with Endoscopy being an indispensable tool, especially for allowing tissue sampling.
Malignant Biliary Stricture:
- The most common cause of malignant biliary stricture is pancreatic adenocarcinoma. As pancreatic cancer is often diagnosed at an advanced stage, 70% of patients already present with stricture at the time of diagnosis.
- The second most common cause is cholangiocarcinoma, a primary tumor of the biliary duct itself.
Regarding the topography of biliary strictures:
- Distal – pancreatic malignancy should always be the most important consideration, regardless of whether there is an identifiable mass on cross-sectional imaging or not.
- Proximal – suspicion of cholangiocarcinoma should be the most considered.
Benign Biliary Stricture:
- The most common cause is iatrogenic injury, especially post-cholecystectomy or post-liver transplant.
- Several inflammatory conditions can also result in strictures, mainly Primary Sclerosing Cholangitis and IgG4-related Sclerosing Cholangitis. However, as most biliary strictures turn out to be malignant upon investigation, it is always essential to rule out malignancy in these patients.
Indeterminate Biliary Stricture (IBS):
- Concept: in most publications, it is postulated that the term refers to a stricture whose etiology has not been established by radiology, whether Computed Tomography or Magnetic Resonance Imaging (due to the fact that there is no obvious mass on cross-sectional images) AND ERCP with transpapillary biopsy and/or cytological brushing also failed to elucidate.
- Note that we highlighted the “AND” because, despite being the most commom definition understanding, there are disagreements on whether to consider that these two steps would be additive.
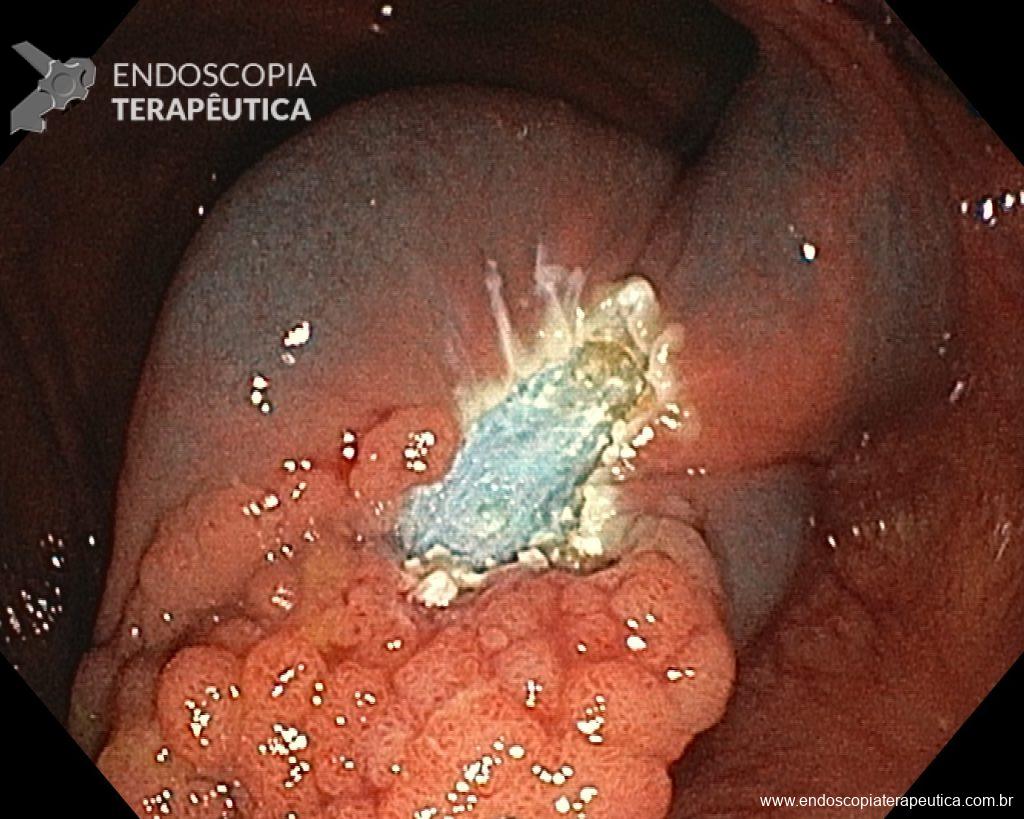
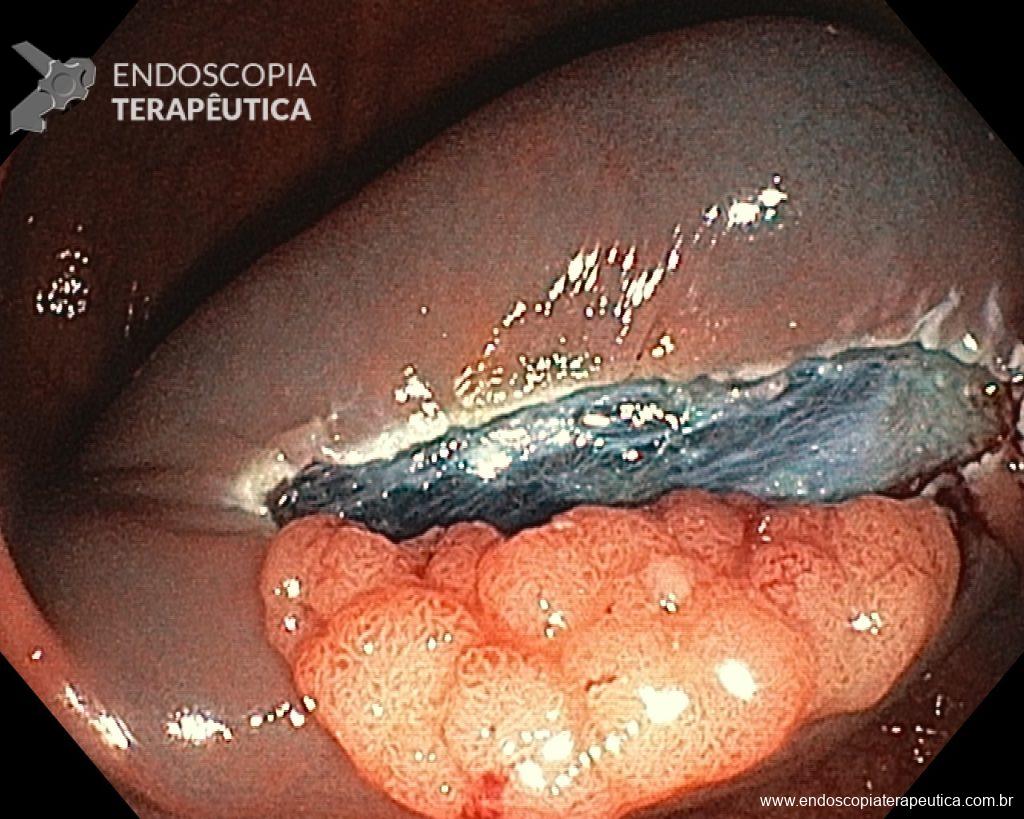
- However, it is a fact that ERCP with transpapillary biopsy (guided by fluoroscopy) and/or brushing continues to be the standard procedure in many services in these cases, especially due to the unavailability of Cholangioscopy. However, given the currently published and very promising results with direct biopsies guided by Cholangioscopy, there may be a change in this diagnostic sequence.
IBS continue to present a diagnostic challenge for physicians and represent 20% of all biliary strictures after initial evaluation. Although most biliary strictures are considered malignant, up to 20% of surgical specimens are found to be benign, thus, incorrect diagnosis can lead to unnecessary surgeries or delays in treatment (when actually malignant).
A brief overview of the most accessible technologies that can help establish the etiology of indeterminate strictures
ERCP with cytological brushing and/or transpapillary biopsy guided by fluoroscopy:
- continues to be the initial approach for evaluation, however the diagnostic yield is often disappointing
- Navaneethan et al. conducted a meta-analysis showing that the sensitivity of cytological brushing is 45%, with a specificity of 99% for the diagnosis of malignant strictures, while the sensitivity of biopsy was 48.1% and the specificity was 99.2%. The combination of the two methods only increases the sensitivity to 59.4%. Similarly, recent prospective studies have shown a sensitivity of 46-56% and specificity of 100% with associated biopsies and brushing. This suggests that both brushing and biopsy result in similar accuracy, and the combination of strategies only modestly increases sensitivity.
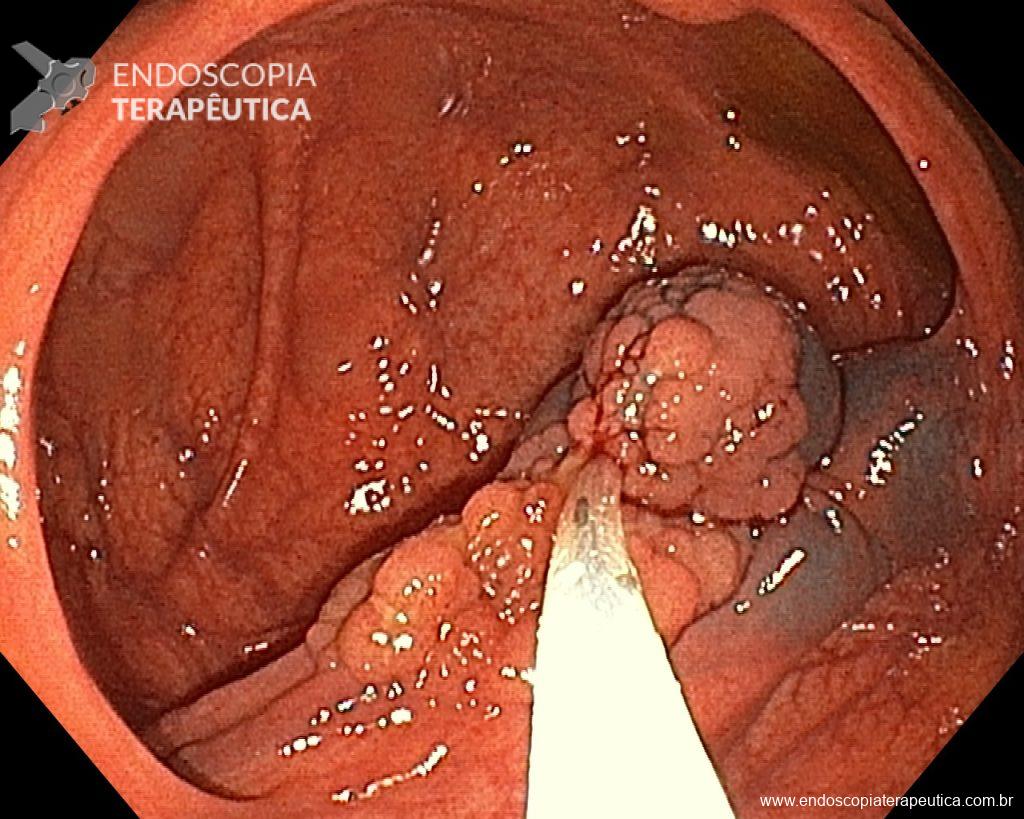
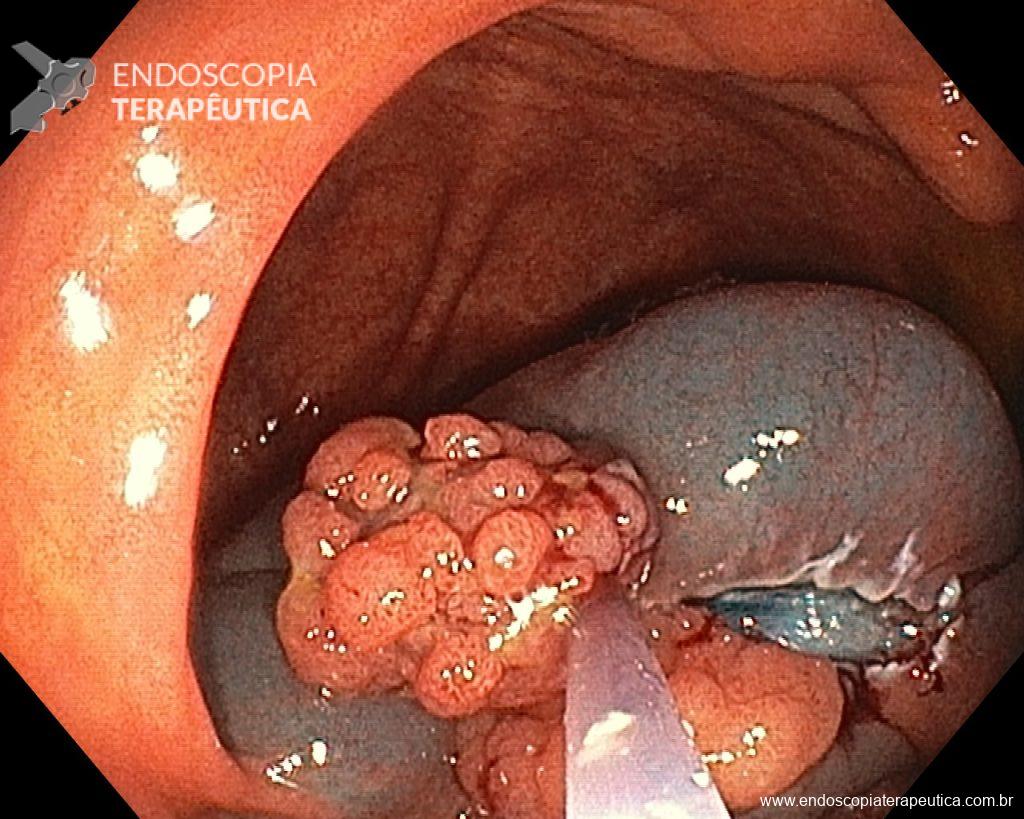
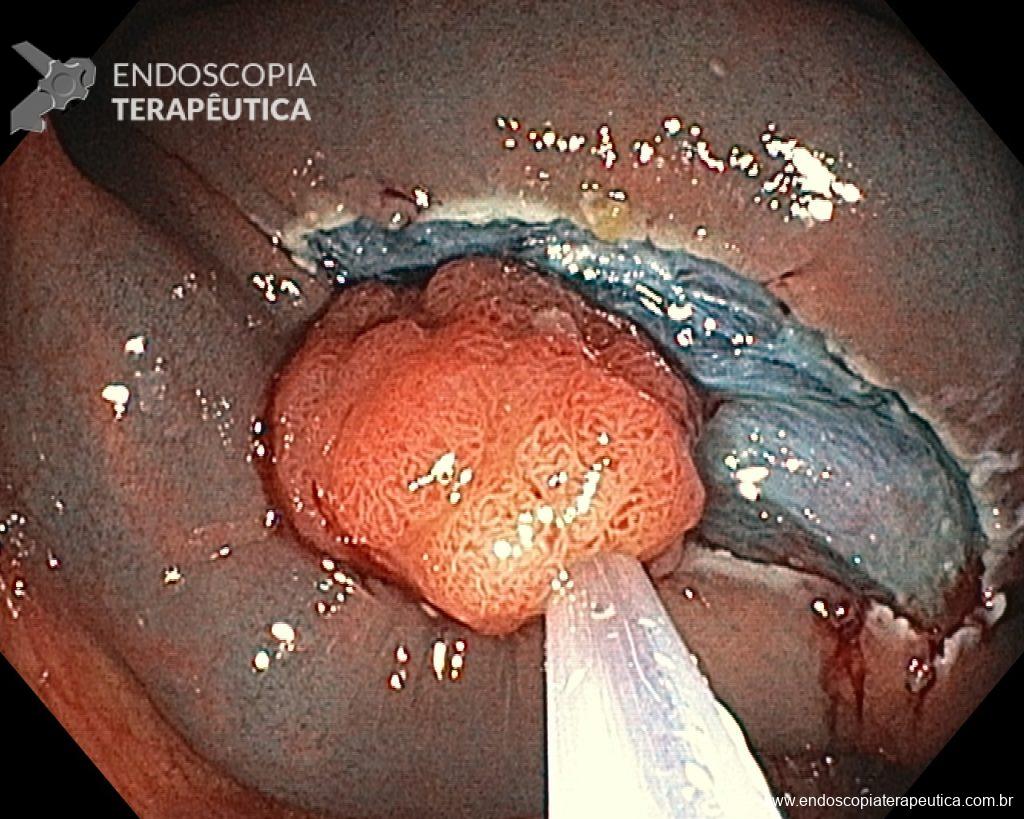
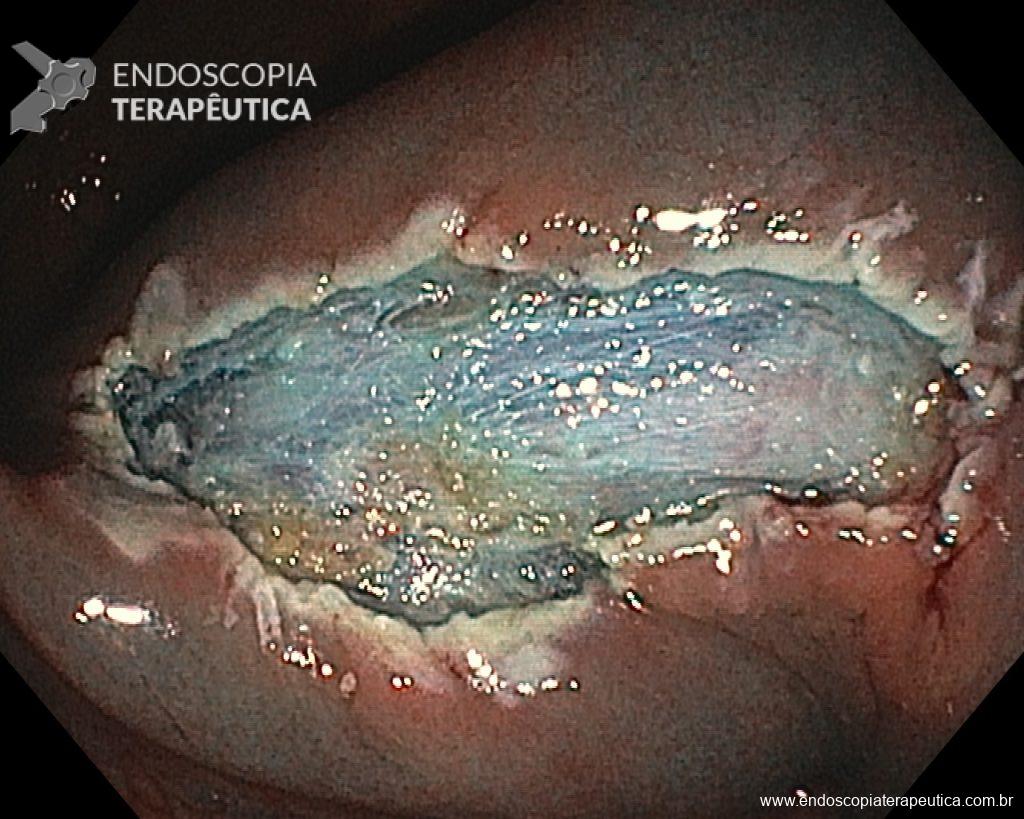
Cholangioscopy:
- The direct Cholangioscopy system was introduced in 2007, being developed for single-operator examination. Currently, there are cholangioscopes with better image resolution, visibility, better maneuverability, and versatility in the use of accessories.
- A recent randomized controlled study (Gerges et al.) of 57 patients compared the use of single-operator digital cholangioscopy with direct biopsies to ERCP with brushing in patients with IBS. The sensitivity of Cholangioscopy was 68.2% vs. 21.4% for ERCP with brushing (p < 0.01). Similarly, a meta-analysis by Navaneethan et al. of 10 studies (456 patients) calculated a combined sensitivity of 60.1% and specificity of 98% with biopsies guided by Cholangioscopy.
Endoscopic Ultrasound (EUS)
- Unlike other endoscopic modalities, EUS also offers the opportunity to evaluate the pancreatic parenchyma and regional lymphadenopathy for potential FNA. It has been frequently used for the evaluation of diseases of the biliary tree and its surrounding structures.
- In two systematic reviews, the sensitivity of fine needle aspiration guided by EUS after a non-diagnostic ERCP was 77% and 89%, with a specificity of 100%. A meta-analysis by Chiang et al. of 10 studies (1,162 patients) showed a 14% increase after a non-diagnostic ERCP. The diagnostic yield of EUS depends in part on the location of the stricture. A 2016 meta-analysis of 13 studies reported a combined sensitivity of 83% for fine needle aspiration of distal biliary strictures compared to a combined sensitivity of 76% for proximal biliary strictures.
Despite current technology aiding in defining the etiology of IBS in many cases, there are still some others considered “negative” during the investigation, but which persist with suspicion of malignancy and end up undergoing surgery. Thus, new technologies are expected to provide not only high diagnostic precision but also a high negative predictive value, precisely to avoid unnecessary surgeries. This is especially true for Cholangioscopy, whose endoscopic criteria are not yet fully established and there is a lack of consensus on terminology and description of findings, which is why histology continues to be the gold standard for diagnosis.
References
- Nichol S. Martinez et al. Determining the Indeterminate Biliary Stricture: Cholangioscopy and Beyond. Current Gastroenterology Reports (2020) 22:58.
- Robert Dorrell et al. The Diagnostic Dilemma of Malignant Biliary Strictures. Diagnostics 2020, 10, 337.
- Chencheng Xie et al. Indeterminate biliary strictures: a simplified approach. Expert Review Of Gastroenterology & Hepatology, 2018.
- Petko Karagyozov et al. Role of digital single-operator cholangioscopy in the diagnosis and treatment of biliary disorders. World J Gastrointest Endosc 2019 January 16; 11(1): 31-40. DOI: 10.4253/wjge.v11.i1.31.
How to cite this article
Brasil, G. Indeterminate biliary stricture – important concepts you need to know. Endoscopy News 2022. Available at: https://endoscopy.news/general-topics/indeterminate-biliary-stricture-important-concepts-you-need-to-know/