Well-performed endoscopic piecemeal mucosal resection (EPMR) is better than a failed attempt of en-bloc mucosectomy (EMR) – Tips for performing without fear!
A real-life case we encounter during colonoscopy schedules:
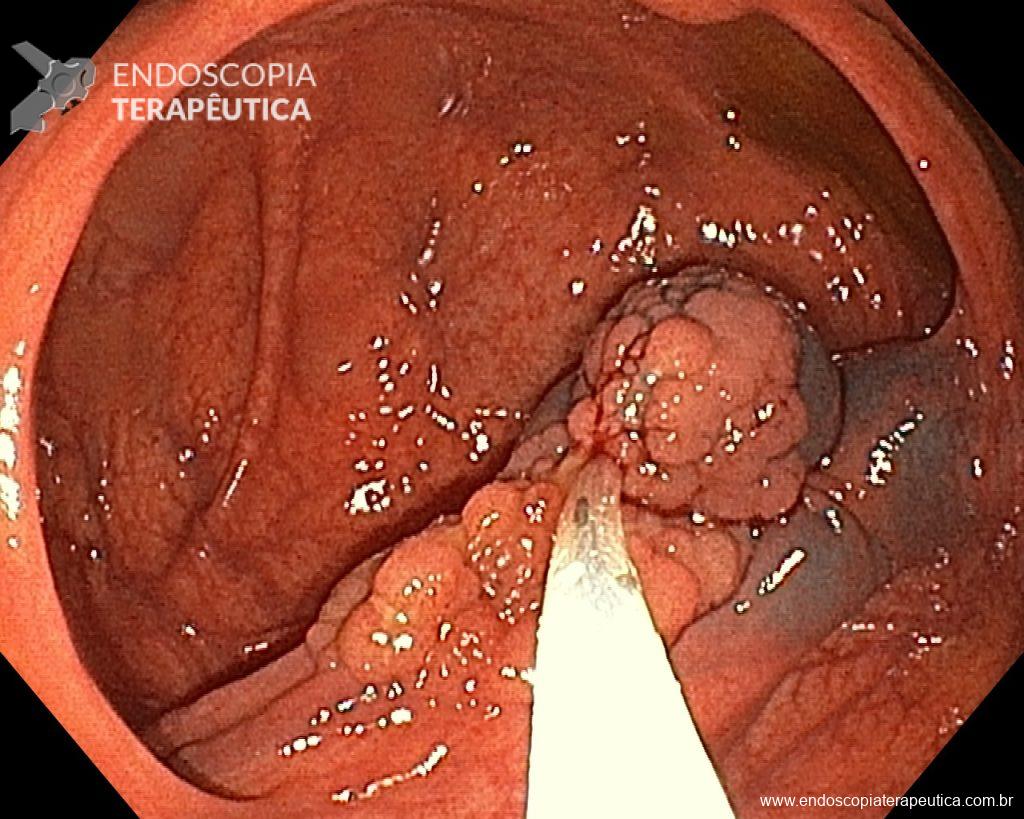
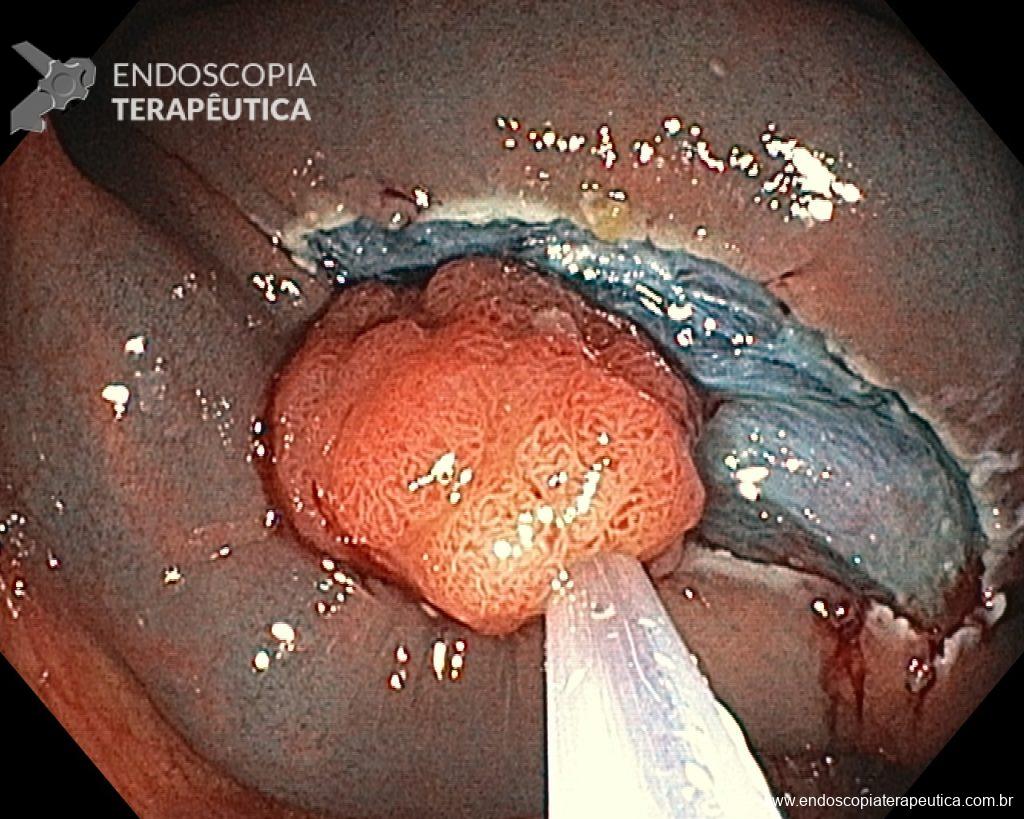
A 60-year-old man, previously healthy, with no comorbidities or relevant family history for gastrointestinal tract neoplasms, underwent his first colonoscopy for colorectal cancer screening/prevention and obtained the following finding:
– Laterally spreading tumor of the granular type, homogeneous (LST-G-H), with regular surface and vasculature on virtual chromoendoscopy with NBI, measuring about 4cm, located in the cecum (Paris 0-IIa / JNET 2A).
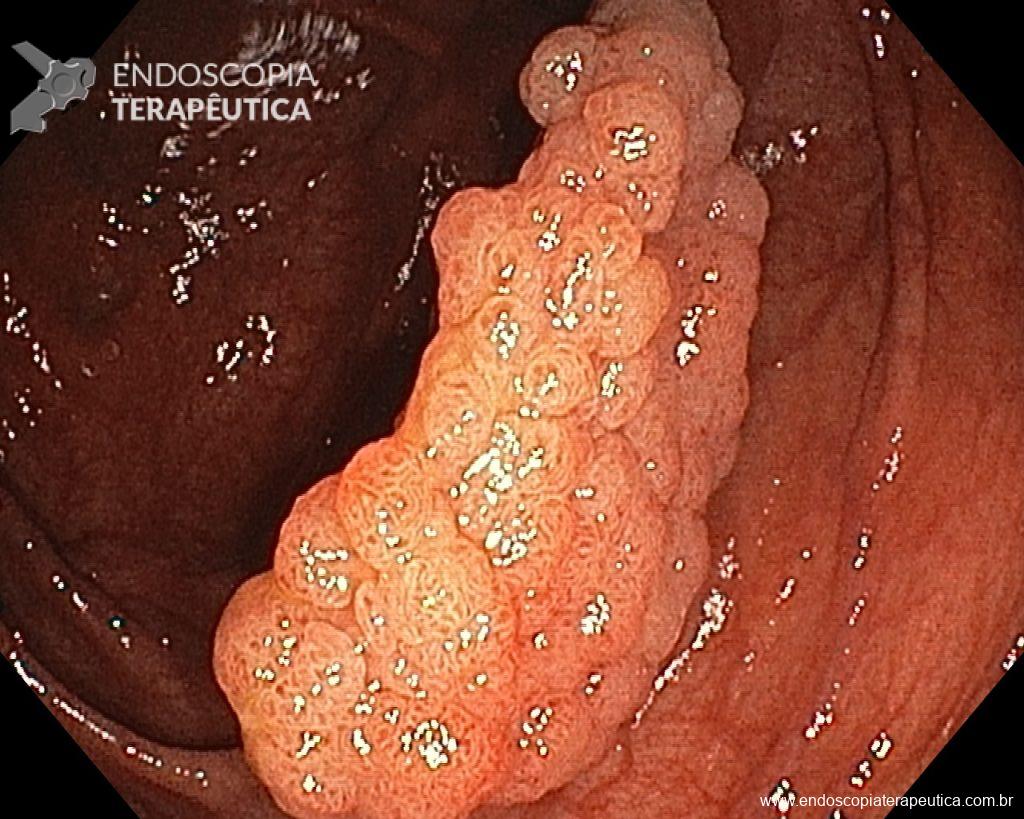
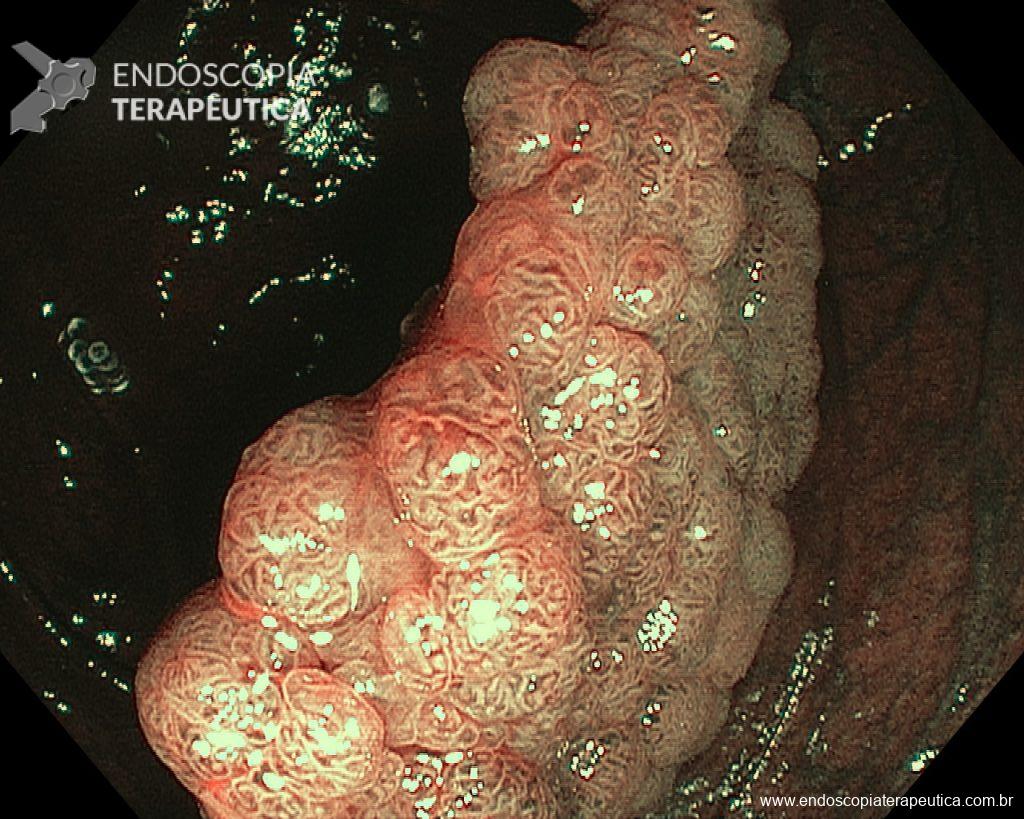
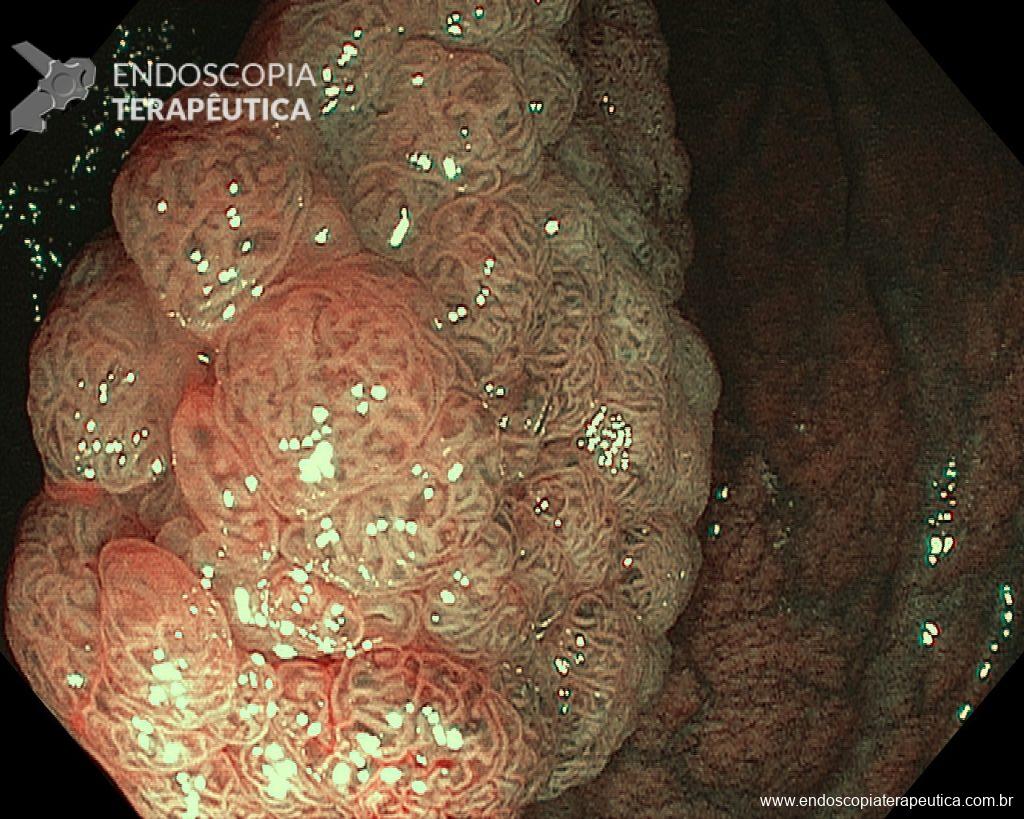
Faced with a lesion of this size, it is natural that doubts arise about the ideal treatment to be offered to the patient, especially in those who do not master advanced endoscopic resection techniques, particularly Endoscopic Submucosal Dissection (ESD).
Classification of LSTs
- The term LST was originally proposed by Kudo et al and describes a pattern of growth of colorectal tumors with a diameter >10 mm, which tend to spread laterally with a smaller vertical axis.
- LSTs can be classified into two types: granular type LST (LST-G), with granules and nodules on the tumor surface, and non-granular type LST (LST-NG), with a flat and smooth surface.
- The former can be subclassified: homogeneous granular type LST (LST-G-H), with granules or nodules uniformly distributed on the tumor surface, and mixed nodular type LST (LST-G-M), with nodules of different sizes on the surface;
- the latter is also subclassified: pseudo-depressed non-granular type LST (LST-NG-PD), when there is a depression in the center of the lesion, and flat-elevated non-granular type LST (LST-NG-F), without depression, that is, completely flat.
learn more about macroscopic classification in this post: click here
Prevalence and location of LSTs
- The estimated frequency of LSTs among all epithelial colorectal tumors, excluding advanced carcinomas, varies in the literature, averaging 4.5%, however, more recent studies with larger case series point to a prevalence that can reach up to 12.3%.
- LST-Gs are the most prevalent, accounting for about 65-70% of all LSTs. While LST-G-Hs tend to be located more in the proximal colon, LST-G-Ms affect the rectum more often and usually reach larger diameters. LST-NGs are more commonly found in the transverse colon.
LST x risk of malignancy
- Each subtype has different and unique characteristics, which requires a decision on the treatment policy to be individualized.
- More than half of LST-G-H lesions are adenomas, with the presence of carcinoma being rare, even when they reach a large diameter, however, even when present, it tends to be restricted to the mucosa (Tis – intramucosal).
- As for LST-NGs, although both LST-NG-F and LST-NG-PD are considered as subtypes, the latter has a higher rate of submucosal invasion even at small sizes, whose nature tends to be multifocal, and it is the subtype with the highest risk among all LSTs. LST-NG-Fs have a low risk of submucosal invasion, and in the few cases present, it tends to be focal.
LST x choice of endoscopic treatment
- ESD is widely used in the upper gastrointestinal tract, however, due to anatomical and histological differences between the colon and the stomach, it is not established as the standard therapeutic technique for colorectal tumors. Furthermore, it is essential to consider, even despite advances in recent years, that the ESD technique is still quite scarce in most medical centers, so the choice of endoscopic treatment technique cannot cause more difficulties than the problem itself.
- Almost all LST-G-Hs do not invade the submucosa. Despite being higher compared to ESD, when well executed, the recurrence rate after EPMR is low, without clinically relevant concerns, since when it occurs it is usually unifocal, small and easily treated in a single session. Therefore, in this morphological type, EMR and EPMR can be adopted as the first option, especially for their higher safety profile.
- Because LST-NG-PDs have the highest rate of submucosal invasion, even at small sizes, with a more multifocal invasive nature and a tendency for greater depth, regardless of whether it is observed only in one location, en bloc resection, mainly by ESD, should always be considered the first option to allow a more reliable pathological evaluation.
- As for LST-G-Ms, most submucosal invasions occur below the largest nodule, however, in up to 17.1% of them, there are also foci of invasion outside the dominant nodule (multifocal invasion). As this morphological type reaches the largest diameters, en bloc resection by EMR is often considered difficult. For these reasons, when opting to perform EPMR, it is necessary to ensure the resection of the dominant nodule in a single piece or proceed with ESD, in order to obtain accurate pathological diagnoses. It is worth remembering that large LST-G-Ms are more frequently located in the rectum, where both the safety profile and the outcomes of a non-curative endoscopic treatment due to the impossibility of histological evaluation (definitive colostomy) favor the performance of ESD over EPMR.
- LST-NG-Fs have a much lower risk of submucosal invasion compared to LST-NG-PDs, in some series even comparable to the risk of LST-G-Hs, therefore, several of those lesions can be cured by endoscopic treatment with EMR or EPMR. However, as the size of this morphological type is associated with a greater possibility of submucosal invasion, especially when larger than 30 mm in diameter, en bloc resection by ESD can also be adopted if they are difficult to remove en bloc by EMR.
Golden tips for performing an EPMR
1. Spend enough time evaluating the lesion to be treated.
Make sure to inspect the lesion to be resected. Make a point of spending enough time evaluating the morphology of the lesion according to the Paris classification, as well as the vascular and glandular patterns. It’s not a waste of time, in fact, you’ll save time by deciding the best way to approach the lesion! Pay attention to the margins, as they may extend beyond the fold. Inspect the lesion with high-definition white light and conventional or virtual chromoendoscopy. A thorough evaluation can identify lesions with possible submucosal invasion and consequently those patients who will benefit from en bloc resection.
2. Do not underestimate the relevance of the lesion’s position.
Have a good position with the device straightened and relaxed. Position the lesion between 5 and 6 o’clock in the endoscopic field. The device and accessory should respond “one to one” to the movements of the hands, fingers, and also the wheels. Working in the best position is extremely effective in minimizing risks and maximizing the outcome of the resection. If a variable stiffness endoscope is being used, take advantage of the potential for retroflexion of the tip. Position the patient so that any fluid or resected pieces accumulate away from the lesion, so that the work field is kept clean and the ideal vision is preserved in case of complication.
3. Choose the snare wisely.
Depending on the morphology or size of the polyp, selecting the most suitable snare can make a difference in the success of the procedure and, therefore, in the outcomes. Small (10–20 mm) or large (25–33 mm) rigid snares with braided wire should be preferred for EPMR and en bloc EMR, respectively. On the other hand, monofilament snares may be the best option for capturing lesions that have difficulty lifting, such as recurrence after EMR or situations where there has been a previous attempt at resection. Use the device as an extension of your hand, placing it parallel to the wall. Adapt the cut to the plane of the lesion, fragment by fragment. The more angle you create between the snare and the wall, the greater the likelihood of involving the muscularis propria. Close the snare well to keep the lesion in place before resecting it. Be aware of the possibility of submucosal fibrosis resulting from previous biopsy collections, previous resection attempts, and LST-NG, as in these situations snare apprehension can be difficult, eventually requiring alternative techniques for lesion removal.
4. Don’t be greedy!
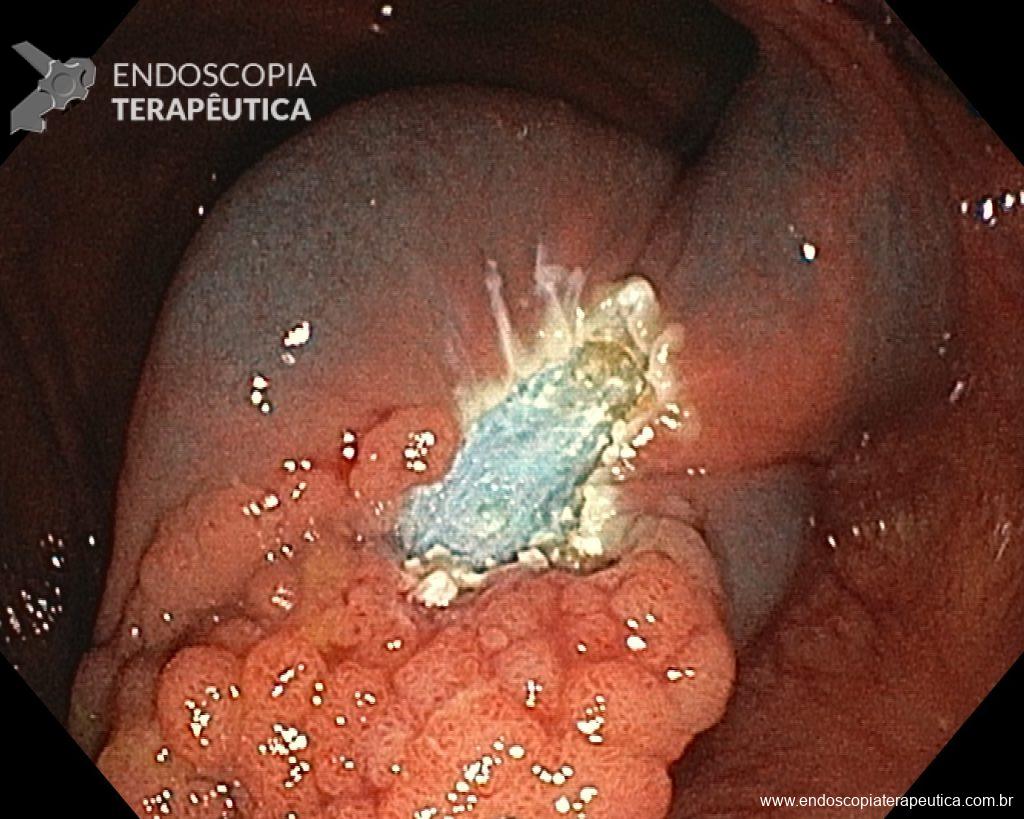
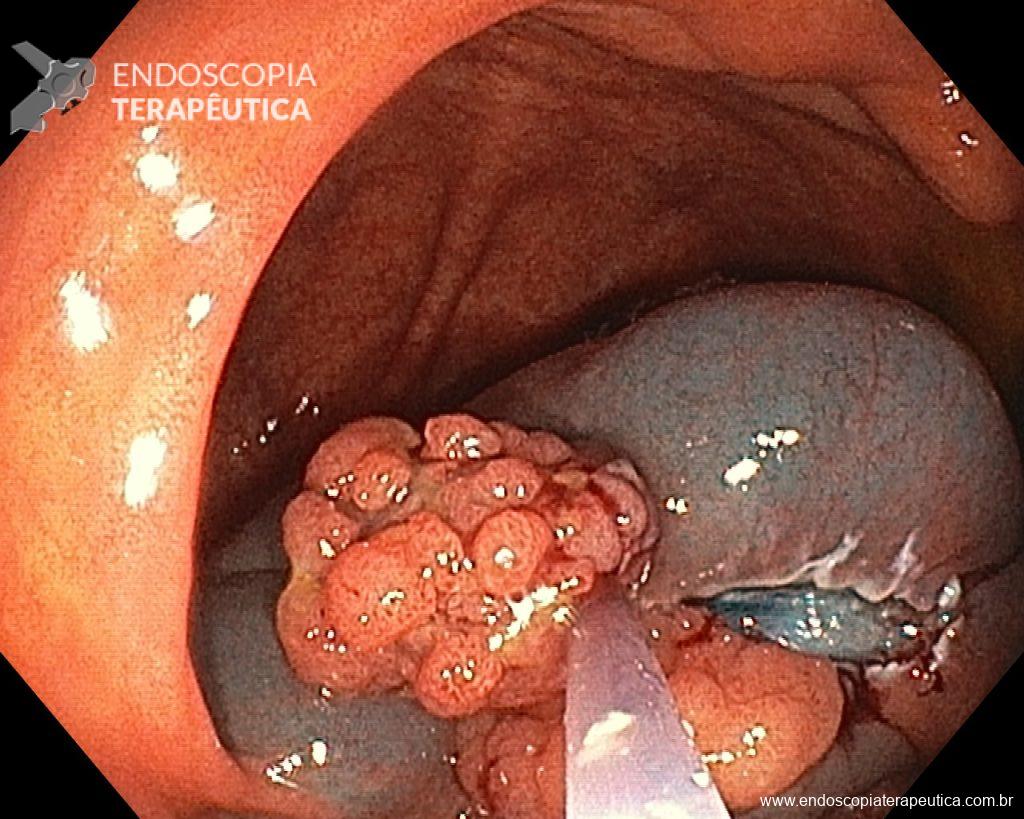
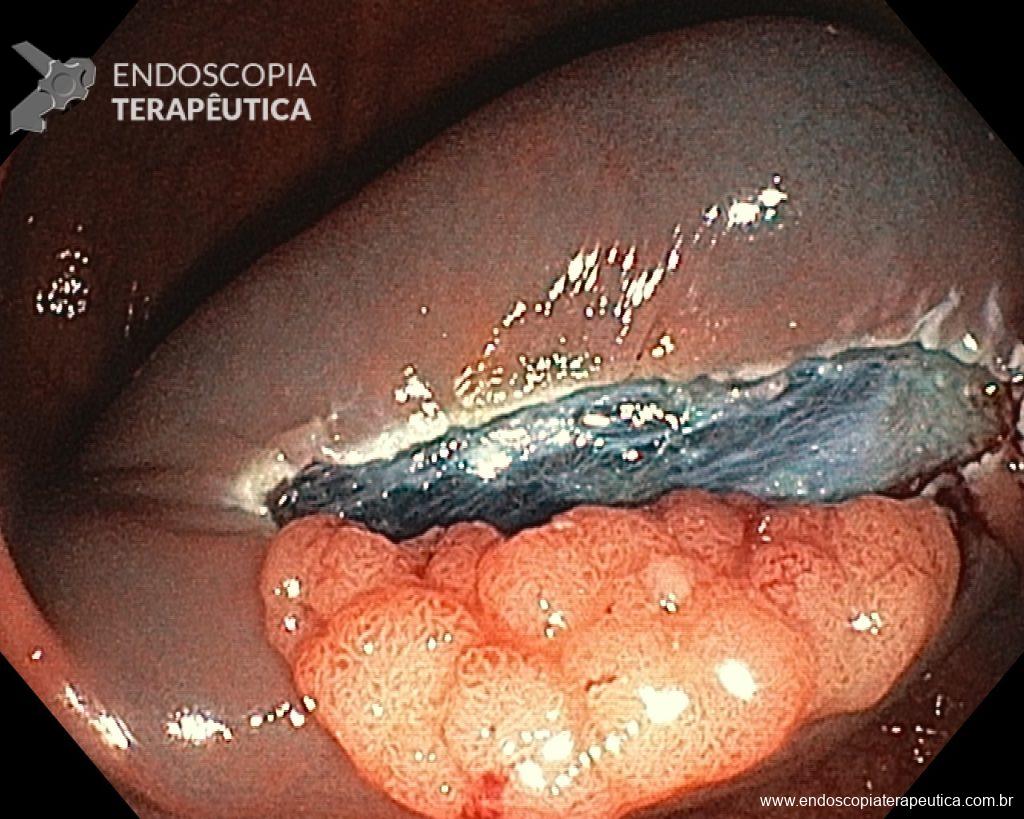
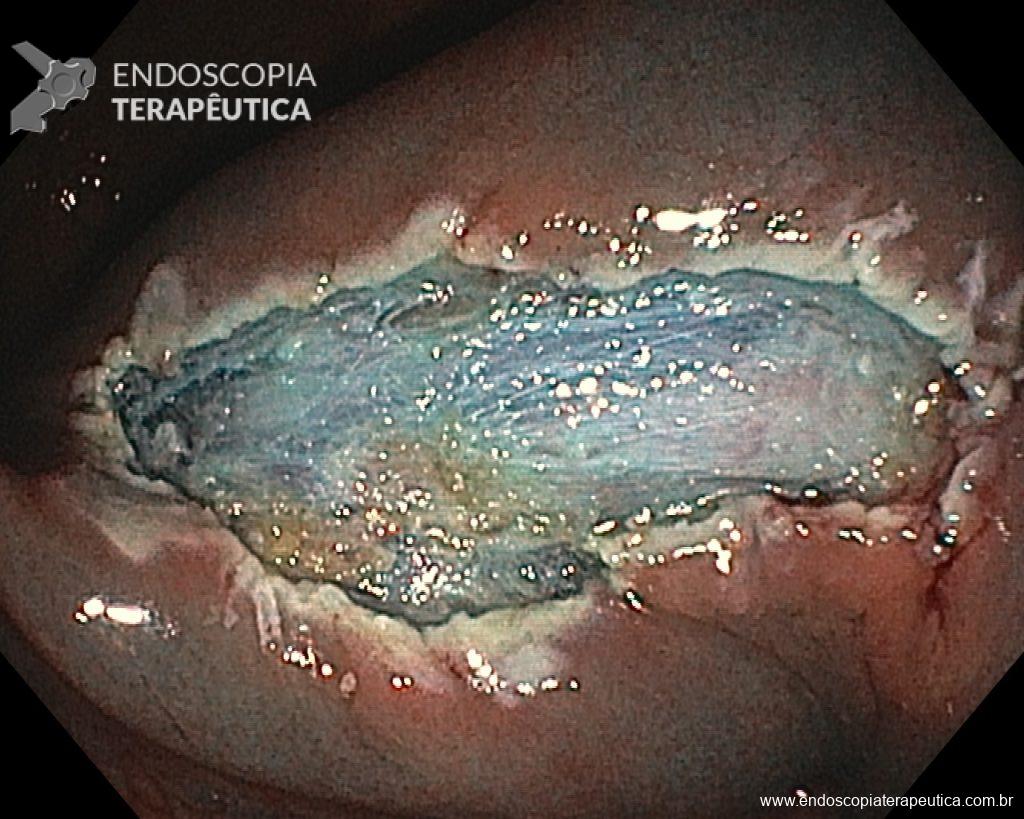
Once the EPMR technique is chosen, keep in mind that the goal should be the complete removal of the lesion with the highest possible safety. For this, the correct strategy is fundamental: do not make the submucosal bubble all at once, instead, make successive injections followed by cutting, preferably in the proximal-distal direction; whenever available, prefer viscous solutions, which ensure greater patency of the bubble; use a smaller snare (10-15 mm) to grasp the formulated bubble, in addition to facilitating, it reduces the risk of perforation when trying to inadvertently grasp the entire lesion.
5. Do not panic with bleeding.
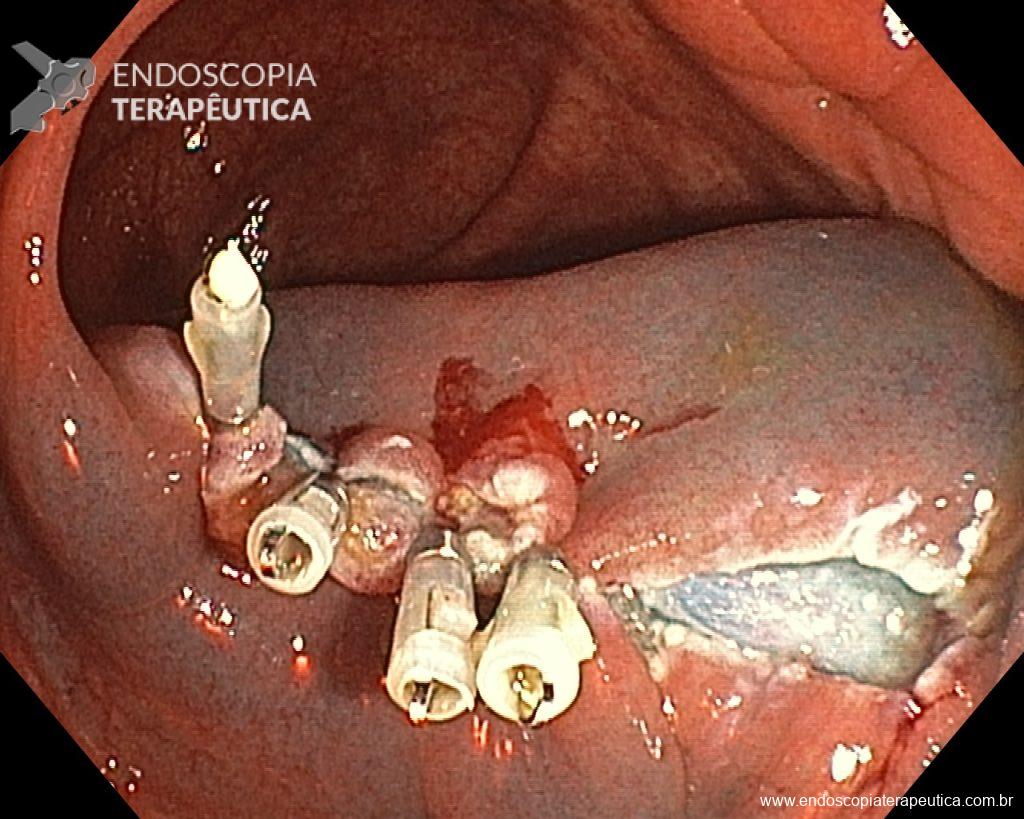
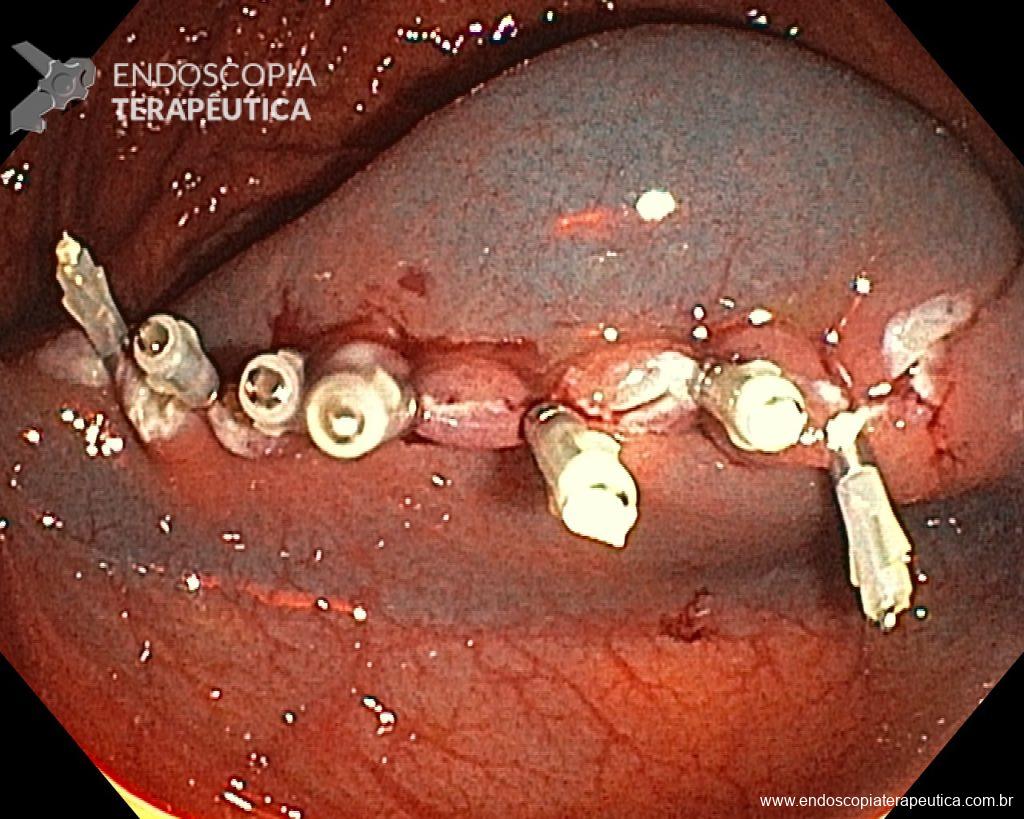
When intraprocedure bleeding (IPB) occurs, do not panic – it’s just bleeding. Although it is true that only practical experience can make you confident in the face of IPB, be prepared to approach it systematically, as you would with any other endoscopic procedure. Before starting the procedure, you must ensure that your endoscopy set is fully equipped and capable of dealing with all types of IPB. Make conscious use of everything you can, without panicking. Use the irrigation pump to remove blood from the target tissue and clean the point where you need to intervene. If you judge that the vessel is small (up to about 2 mm), you can coagulate it immediately with the tip of the snare in “soft coagulation” mode. On the other hand, if the vessel is larger than 2 mm, the use of a coagulation forceps is a more effective strategy. While waiting for the accessory, if you are using a cap attached to the tip of the device, use it as a “finger” and press on the vessel. When you are ready with your device, use the water pump again to clean the area, open the forceps and grasp the vessel, pulling it towards you (and away from the wall) before coagulation. The use of argon plasma coagulation (APC) during EMR should be minimized, as well as hemostatic clips, which should be used when you have tried everything and the bleeding continues.
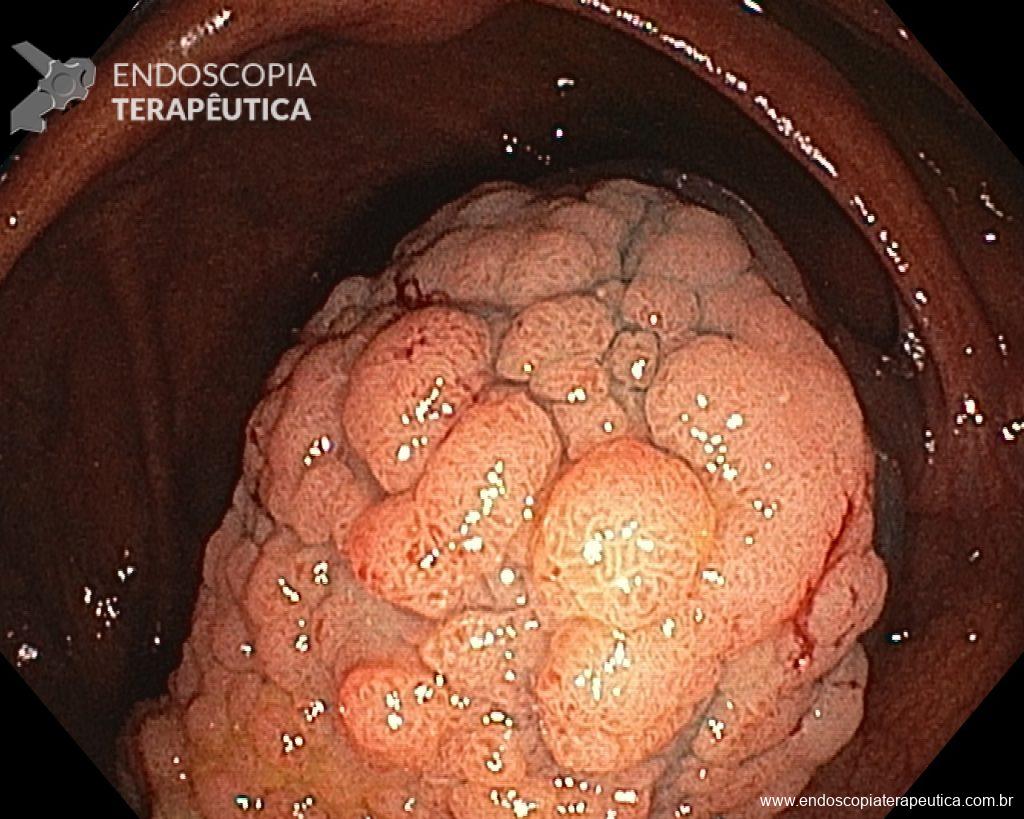
Given the above, for the case exemplified above, it was decided to proceed with resection using the EPMR technique, both for the morphological type (LST-G-H) and characteristics of the lesion surface (JNET 2A), as well as for the location (cecum), which presents a higher risk of complications.
References
- Papparella L et al. Efficacy and safety of endoscopic resection techniques of large colorectal lesions: experience of a referral center in Italy. Eur J Gastroenterol Hepatol 2022; 34: 375–381.
- Ishigaki T et al. Treatment policy for colonic laterally spreading tumors based on each clinicopathologic feature of 4 subtypes: actual status of pseudo-depressed type. Gastrointest Endosc 2020; 92: 1083-94.
- Auriemma F and Repici A. Mistakes in endoscopic resection and how to avoid them. UEG Education 201