Role of Endoscopic Ultrasound in Idiopathic Acute Pancreatitis
Introduction
Acute pancreatitis is one of the diseases responsible for the highest number of emergency hospitalizations in gastroenterology. Its incidence is 13 to 45 cases per 100,000 people, accounting for 270,000 hospitalizations per year in the United States 1. Most cases are mild, but it can evolve into severe forms, requiring intensive care unit admission and even death. Its mortality is close to 5%, being significantly higher when only the most severe cases are analyzed 2.
Gallstone disease and alcoholism are the main causative agents, accounting for about 60% to 80% of cases 3. Other less common causes include anatomical changes, metabolic disorders, tumors, autoimmune diseases, among others. However, in a significant percentage of cases, about 10% to 30%, it is not possible to identify a causative factor after initial evaluation 3-4. It is then defined as idiopathic acute pancreatitis, being the 3rd most common cause in some series 3.
A detailed evaluation in these patients with idiopathic acute pancreatitis is of fundamental importance, since 14% to 26% may present recurrent episodes, evolving to chronic pancreatitis 5-6. In some cases, after specialized examinations, a treatable causative agent can be identified, thus avoiding new crises.
The endoscopic ultrasound is a minimally invasive procedure and, due to the proximity of the stomach and duodenum to the pancreas and bile ducts, allows a detailed examination of this region. Several studies have shown its benefit in the investigation of patients with idiopathic acute pancreatitis, however, the timing of its performance is not yet well defined 1,2-8.
Accuracy of Endoscopic Ultrasound
The accuracy of endoscopic ultrasound in identifying a causative agent in patients with idiopathic acute pancreatitis varies greatly among studies, from 29% to 88% 4. This large difference is due to the inclusion criteria used in each study. In those where patients underwent a greater number of diagnostic tests before the endoscopic ultrasound, the accuracy was lower. In those where patients were referred earlier for endoscopic ultrasound, the accuracy was higher.
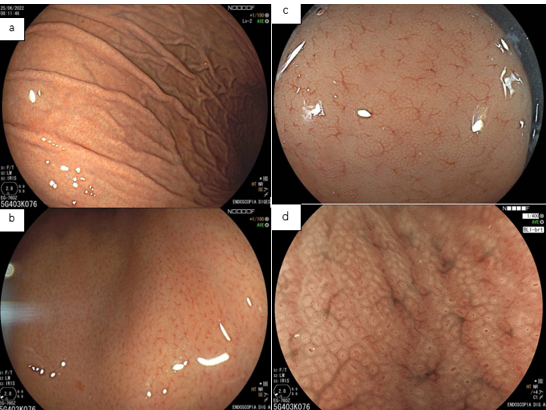
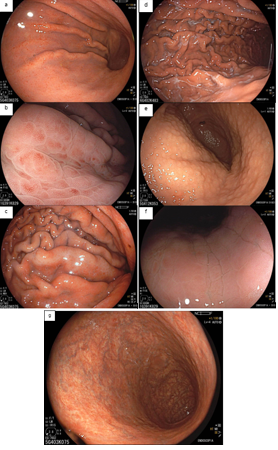
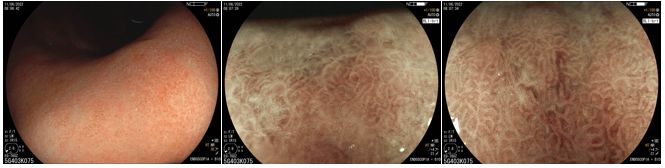
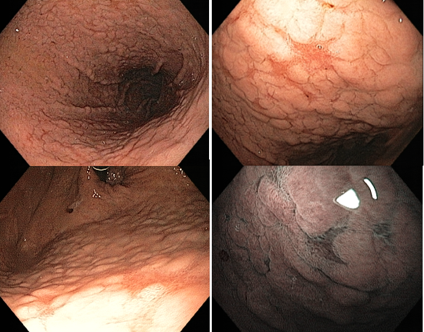
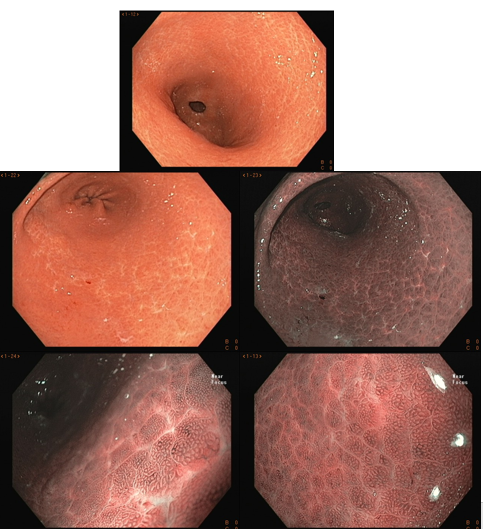
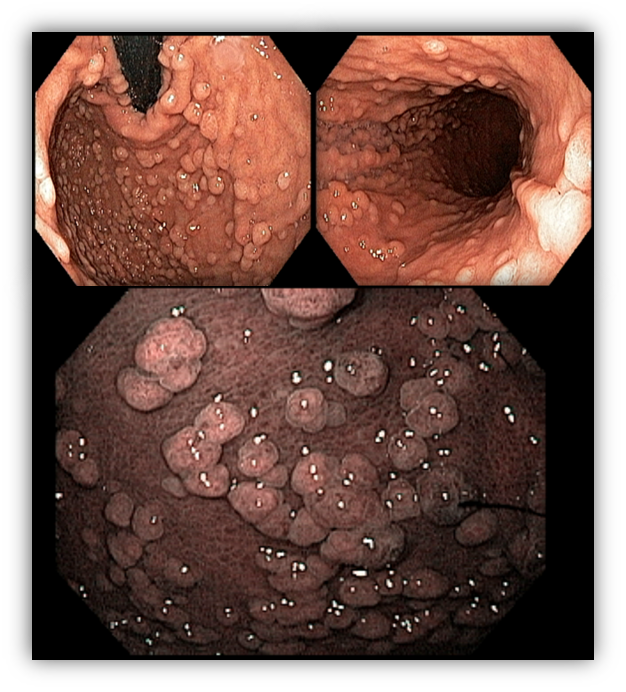
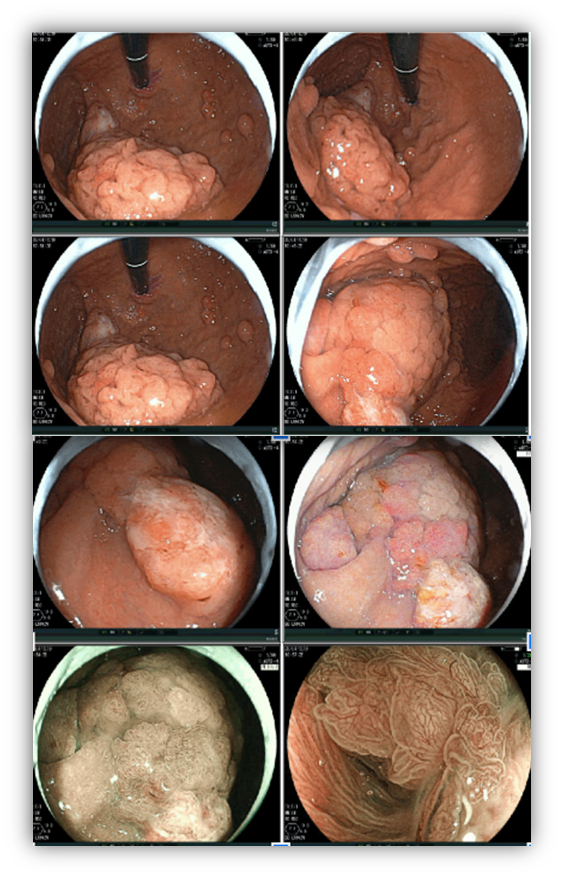
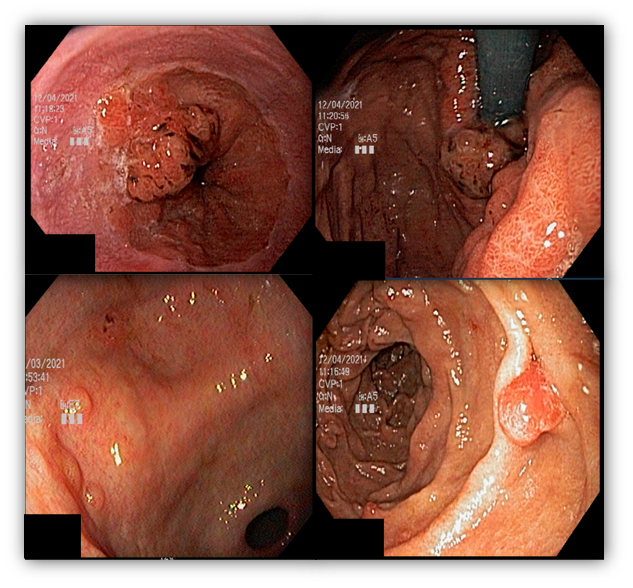
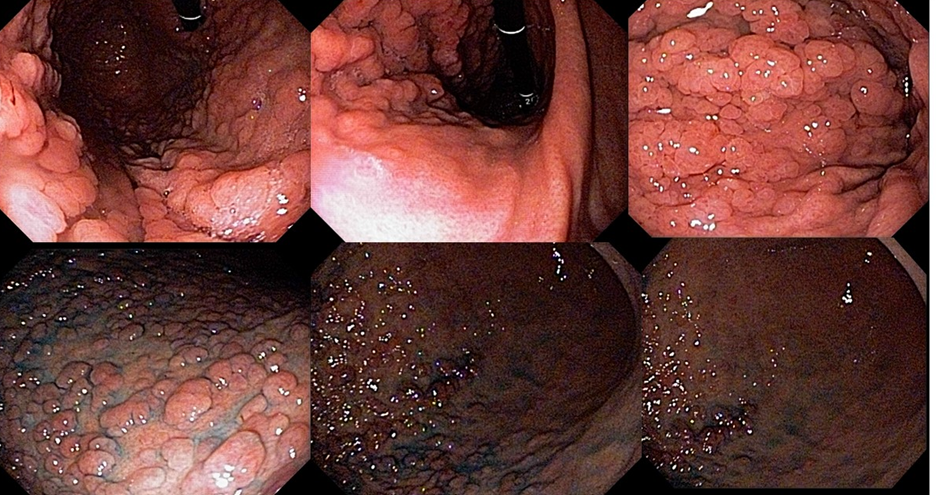
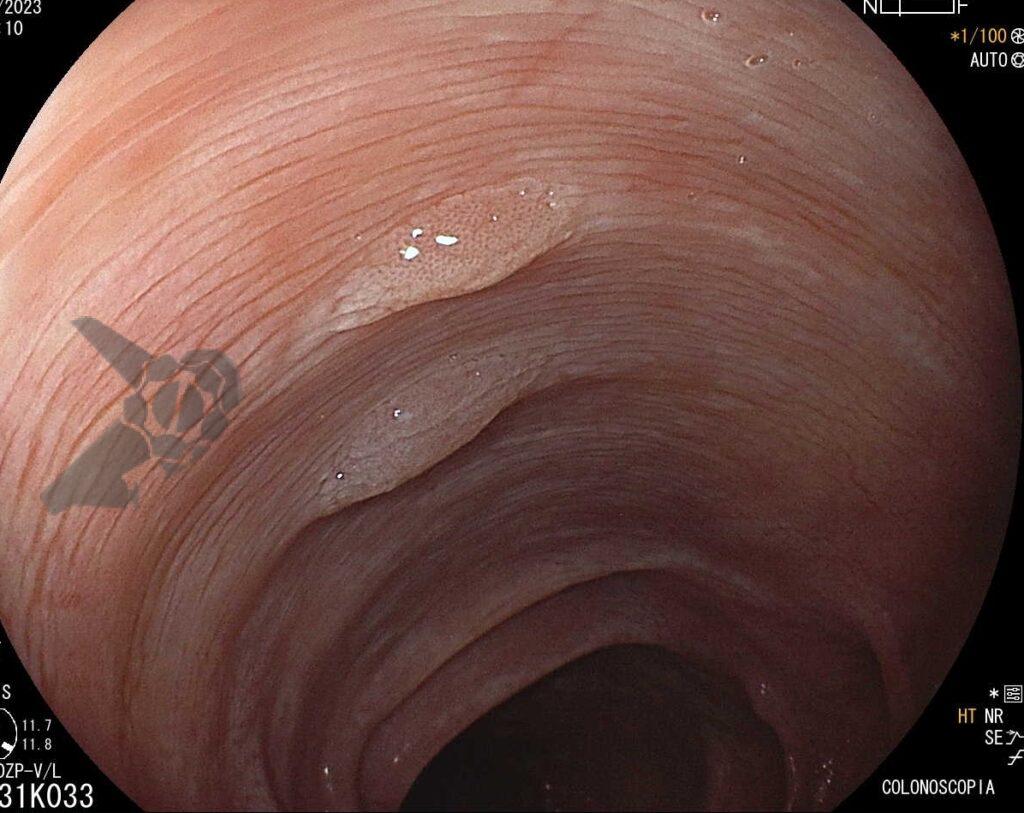
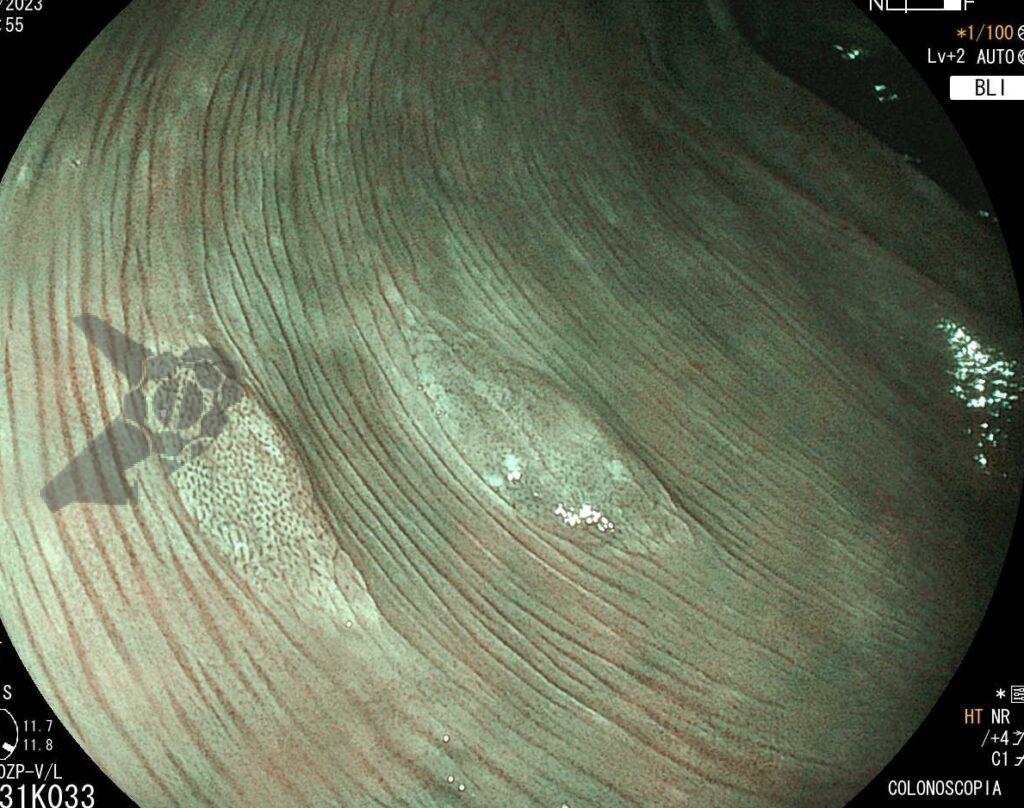
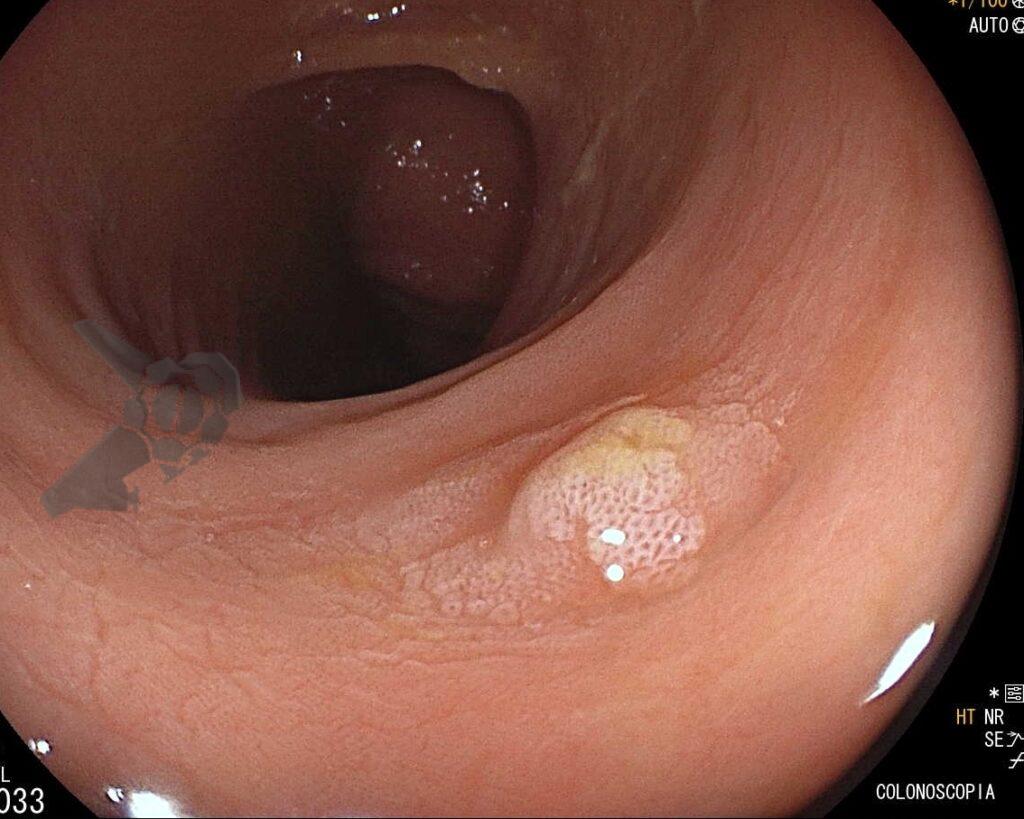
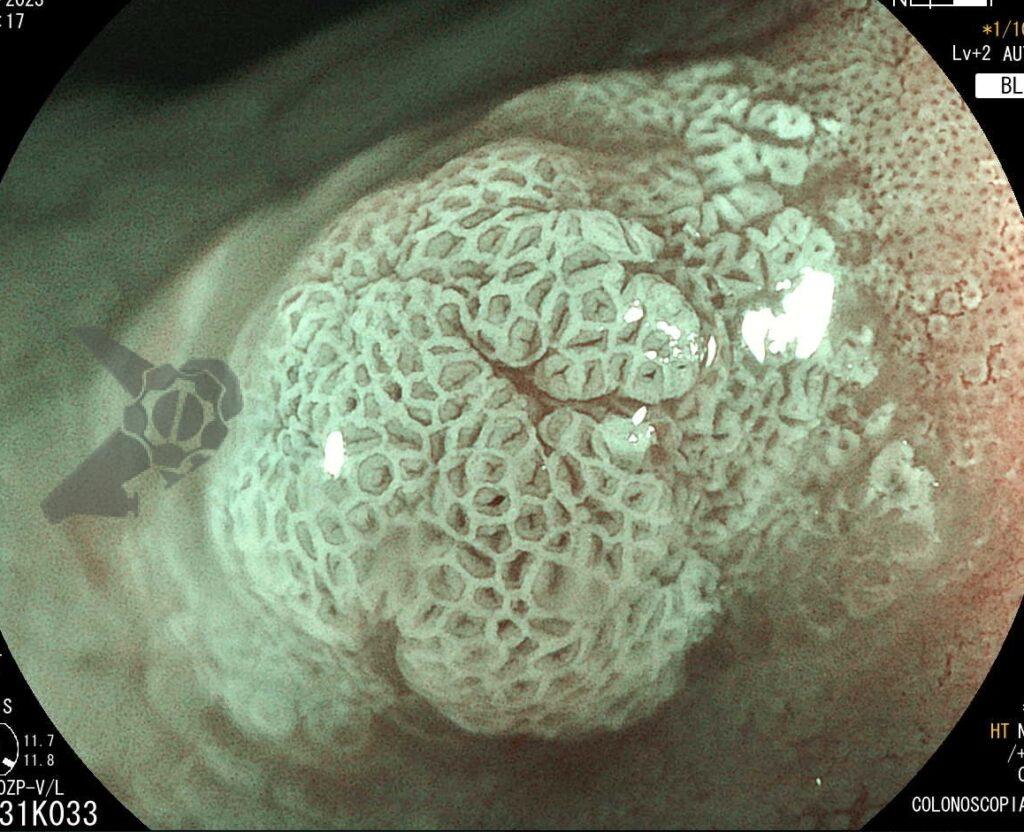
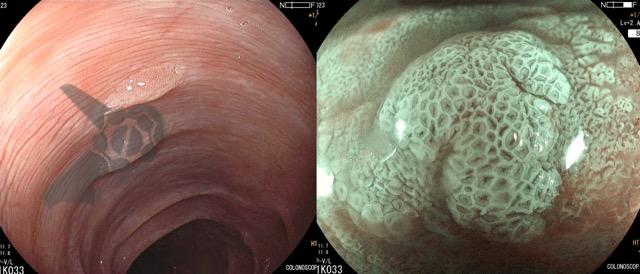
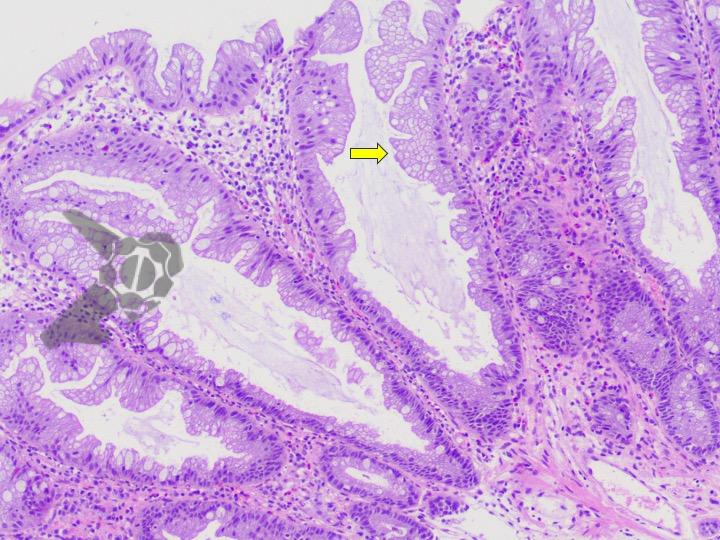
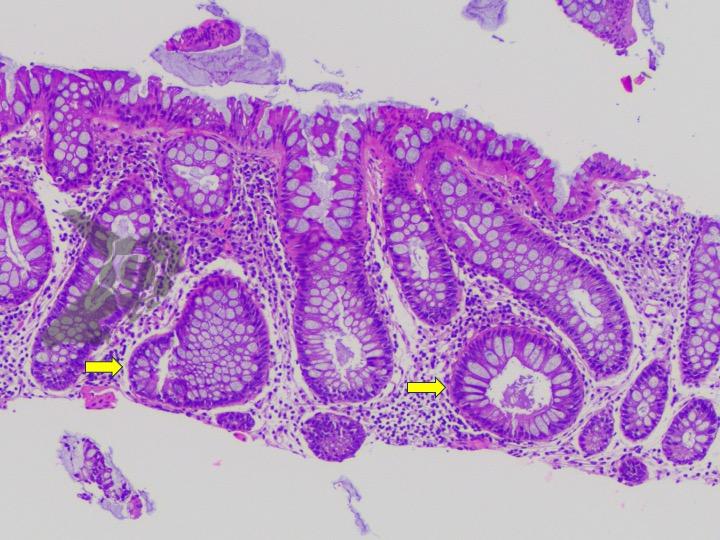
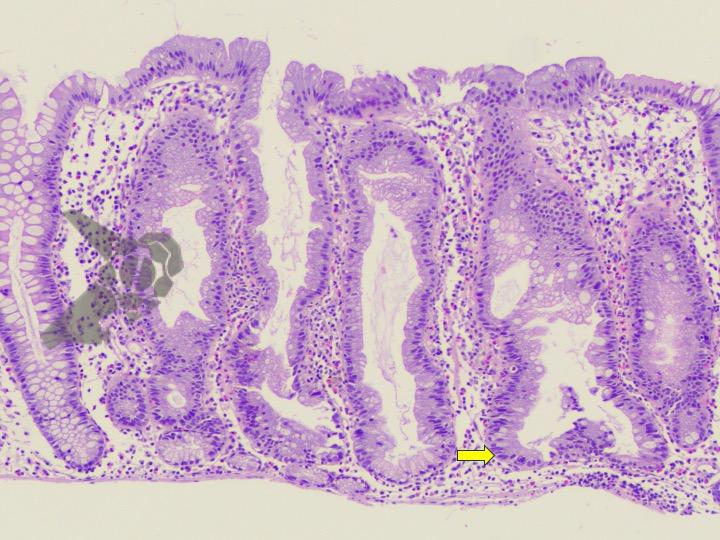
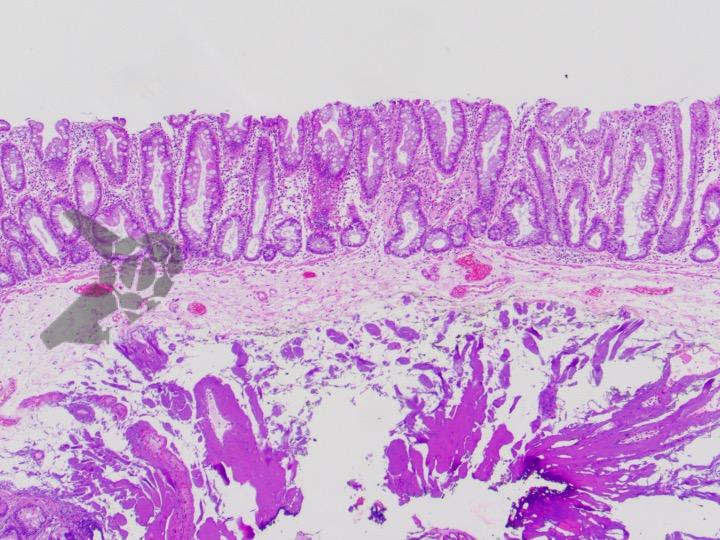
Umans and colleagues in a recent meta-analysis reached an accuracy of 59% 7. Biliary lithiasis, presence of stones, microlithiasis or biliary sludge in the gallbladder or common bile duct, was the most common cause, accounting for 30% of cases (Figures 1 and 2). In second place came chronic pancreatitis with 12% and in third pancreas divisum with 5%. It is important to note that in 2% of patients a neoplasm that had not been diagnosed in previous exams was detected. The identified lesions were intraductal papillary mucinous neoplasms (IPMN), pancreatic carcinomas, neuroendocrine tumors (Figure 3), adenomas, and papillary carcinomas. Other less common causes were autoimmune pancreatitis, ascariasis, choledochocele (Figures 4 and 5), biliopancreatic junction anomaly, and diverticulum.
In the same meta-analysis, when comparing the accuracy of endoscopic ultrasound in patients who had previously undergone cholecystectomy with those who had not, the result was different between the two groups, being 50% and 64%, respectively 7. Thus demonstrating how gallbladder lithiasis is indeed one of the most common causes.
When to Perform Endoscopic Ultrasound After an Episode of Pancreatitis?
There is controversy in the literature about the ideal timing for endoscopic ultrasound after an episode of acute pancreatitis 5. Authors who suggest performing the procedure earlier, sometimes while the patient is still hospitalized, argue that a possible diagnosis could be made more quickly, avoiding the possibility of recurrence and also preventing the patient from losing follow-up 5. Those who prefer to perform the procedure later, about 4 weeks after the resolution of the case, argue that the inflammatory changes secondary to pancreatitis could complicate the diagnosis, reducing the accuracy of endoscopic ultrasound 5.
In the meta-analysis by Umans and colleagues, the accuracy of endoscopic ultrasound after the improvement of acute pancreatitis and before improvement was 61% and 48%, respectively 7.
Perform Endoscopic Ultrasound After the First Episode of Idiopathic Acute Pancreatitis or Only in Recurrent Cases?
There is no consensus in the literature on the ideal indication for performing endoscopic ultrasound 5. There seems to be a similar accuracy when performed after the first episode or after recurrent episodes 5,7. Since many of the identified causes are treatable and would prevent new crises, there is a tendency to already indicate endoscopic ultrasound after the first episode.
Endoscopic Ultrasound vs. MRCP
A meta-analysis by Wan and colleagues, comparing the accuracy of endoscopic ultrasound with MRCP, showed better performance with endoscopic ultrasound, 64% and 34%, respectively 8. The main benefit occurred in biliary lithiasis (34% x 9%) and chronic pancreatitis (10% x 1%). In pancreas divisum, the accuracy was similar with both techniques (2% x 2%). When secretin was used, which is not available in Brazil, MRCP was better (12%). Hallenslebem and colleagues demonstrated similar accuracy between endoscopic ultrasound (36%) and MRCP (33%) 8.
Conclusion
Endoscopic ultrasound plays a fundamental role in the investigation of patients with idiopathic acute pancreatitis. It presents high accuracy for the diagnosis of causative factors, many of which are treatable, thus avoiding recurrent crises.
It is not yet well established in the literature what would be the ideal timing for the procedure, but most studies tend to wait about 4 weeks after the improvement of pancreatitis for its performance, thus minimizing the diagnostic difficulty secondary to inflammatory changes. Most authors also recommend performing endoscopic ultrasound after the first crisis. It is important to note that the diagnosis of neoplasms not detected by other methods can reach 7% 6.
MRCP and endoscopic ultrasound should be used in conjunction. Since biliary lithiasis would be the most common cause and endoscopic ultrasound would have better accuracy for this diagnosis, there is a tendency to indicate it as the first option after an initial negative investigation.
References
- Working Group IAPAPAAPG. IAP/APA evidence-based guidelines for the management of acute pancreatitis. Pancreatology 2013; 13: e1–e15.
- Crockett SD, Wani S, Gradner TB, et al. American Gastroenterological Association Institute Guideline on Initial Management of Acute Pancreatitis. Gastroenterology 2018; 154(4): 1096-1101.
- Blanco GDV, Gesuale C, Varanese M, et al. Idiopathic acute pancreatitis: a review on etiology and diagnostic work-up. Clin J Gastroenterol 2019; 12(6): 511-524.
- Tepox-Padrón A, Bernal-Mendez RA, Duarte-Medrano G, et al. Utility of endoscopic ultrasound in idiopathic acute recurrent pancreatitis. BMJ Open Gastroenterol 2021; 8(1): e000538.
- Somani P, Sunkara T, Sharma M. Role of endoscopic ultrasound in idiopathic pancreatitis. World J Gastroenterol 2017; 14: 6952-6961.
- Hallensleben ND, Umans DS, Bouwense SA, et al. The diagnostic work-up and outcomes of “presumed” idiopathic pancreatitis: A post-hoc analysis of a multicentre observational cohort. United European Gastroenterol J. 2020; 8(3): 340-350.
- Umans DS, Rangkuti CK, Weiland CJS, et al. Endoscopic ultrasonography can detect a cause in the majority of patients with idiopathic acute pancreatitis: a systematic review and meta-analysis. Endoscopy 2020; 52(11): 955-964.
- Wan J, Ouyang Y, Yu C, et al. Comparison of EUS with MRCP in idiopathic acute pancreatitis: a systematic review and meta-analysis. Gastrointest Endosc 2018; 87(5): 1180–8.
How to cite this article
Retes FA. Role of Endoscopic Ultrasound in Idiopathic Acute Pancreatitis. Endoscopia Terapeutica 2023, Vol II. Available at: https://endoscopy.news/general-topics/role-of-endoscopic-ultrasound-in-idiopathic-acute-pancreatitis/