The Helicobacter pylori
The Helicobacter pylori is a gram-negative bacterium that induces cellular and chemical reactions in the stomach, being considered a human carcinogen. Its diagnosis and treatment play an important role in the prevention of associated diseases, such as gastric cancer, ulcers, MALT lymphoma, and hyperplastic polyps. (To learn more about H. PYLORI, visit this post 1 and this post 2)
There are several diagnostic tests, from non-invasive (serology, breath test, and fecal antigen) to invasive (urease, culture, and histology). Non-invasive methods have high accuracy, but do not assess changes in the gastric mucosa. To learn more about diagnostic tests visit this article.
Due to the focal nature of bacterial colonization, the accuracy of invasive methods depends on the location, number, and size of biopsies. These, when poorly directed, can result in false negatives. Thus, it is important to evaluate the endoscopic predictors of the presence or absence of H. pylori in order to direct biopsies to areas with a higher probability of infection, as well as to avoid them when the positive predictive value is high.
Studies have shown that, although not pathognomonic, some endoscopic findings are associated with the presence of H. pylori. Recently, new chromoendoscopy and magnification technologies have allowed the analysis of the microstructure of the gastric mucosa and, consequently, greater accuracy in determining the infection status (absence, active infection, and post-eradication).
The aim of this article is to assist endoscopists in assessing endoscopic findings related to H. pylori both with white light, as well as chromoendoscopy and magnification.
So let’s go!
2. Findings of the non-infected stomach
2.1 With white light
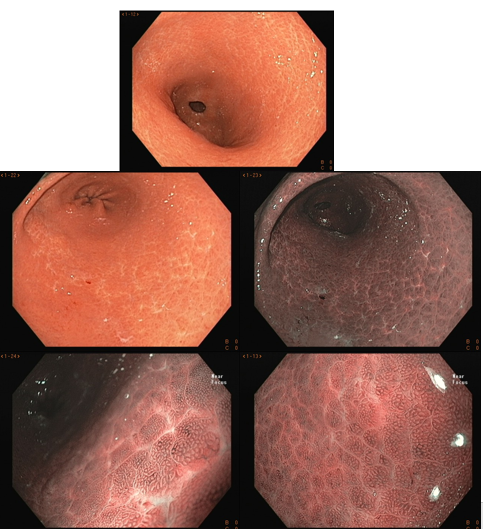
The normal stomach has a pinkish-red and shiny color, and the mucosal folds are present uniformly. The mucus should be hyaline and often forming a small pool. In the body and fundus, the folds are more concentrated on the greater curvature, in a tent shape and tend to disappear with insufflation. The antrum is flat, with a clear hue.
2.2 With chromoscopy and magnification
To understand the changes visualized in endoscopic magnification, we first need to know the histomorphology of the normal gastric mucosa.
To learn more about the normal histology of the stomach Click here
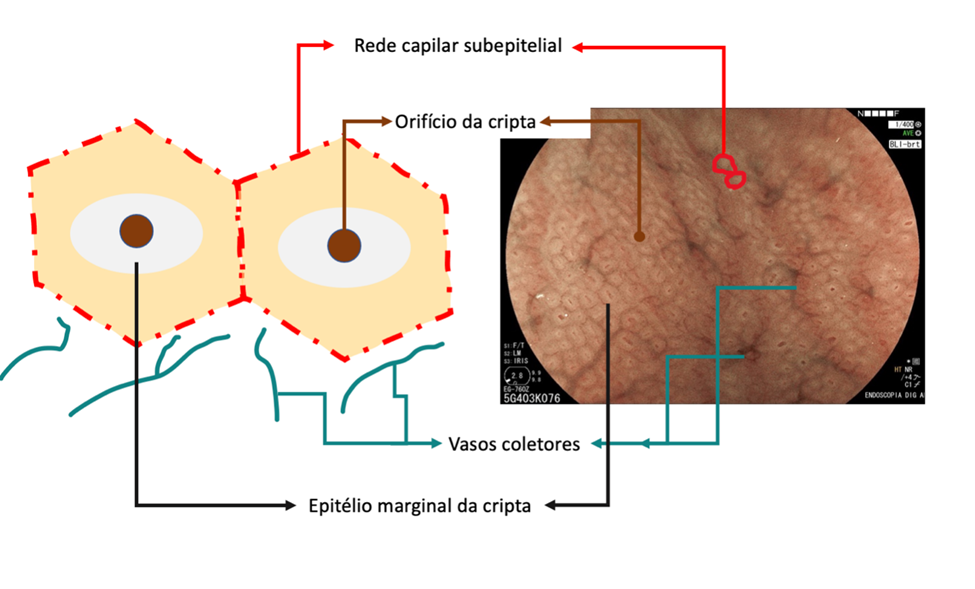
In summary, the mucosa of the gastric body is composed of cryptic orifice (OC), marginal epithelium of the crypt (MCE), subepithelial capillary network (SECN), collecting vessels, and intervening spaces (between the crypts), as shown in the following scheme:
In the stomach not infected by H. pylori, the subepithelial capillary network is present, regularly throughout the body, called RAC (regular arrangement of collecting venules). The negative predictive value of this finding is greater than 90%, which means that its presence in the distal body’s lesser curvature and incisura is strongly associated with the condition of non-infection by H. pylori.
We can also observe that both the crypt orifice and the marginal epithelium are oval, regular, and symmetrical. The subepithelial capillary network (SECN) is regular and fine, in a honeycomb pattern.
3. Findings of the infected stomach
3.1- With white light
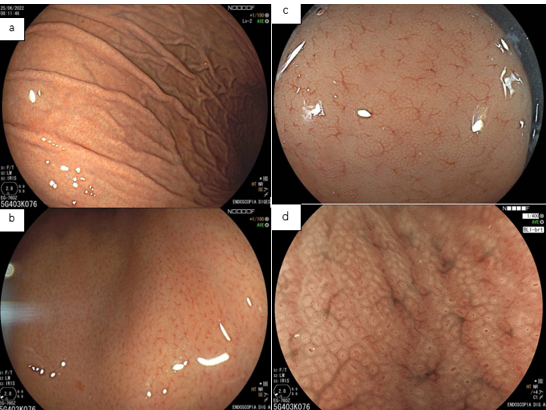
With white light, the endoscopic findings most associated with H. pylori infection are: diffuse hyperemia, petechial redness (“speckled”) of the fundus and proximal body, thickened and tortuous folds, mucosal edema, fibrinous exudate in the body, and antral nodularity. With the persistence of the infection, there is a decrease in the folds and the submucosal vessels become more visible, findings of atrophic gastritis.
In a prospective multicenter study, the sensitivity and specificity of the endoscopic findings described above were 94.3% and 62.8% (KATO,2013). Diffuse hyperemia was considered the most reliable characteristic by experienced endoscopists.
We know that this infection starts in the antrum and progresses to the body. However, in the antrum, the diagnostic accuracy is lower, as the vessels are located more deeply, hindering their visualization. Therefore, we must first assess the presence or absence of hyperemia in the body. When this assessment is difficult, we should pay attention to petechial redness, edema, folds, and fibrinous exudate.
As previously described, the absence of the regular pattern of collecting venules (RAC negative) may be associated with active infection by H. pylori, but the specificity of this finding is low. In a Brazilian study (Fiuza F, Martins BC, 2021), the absence of RAC was associated with only 50.6% positivity of H. pylori. In other words, the absence of RAC has high accuracy for the presence of the bacterium, but the infection is not always what causes its loss.
It is important to remember that, in H. pylori infection, the RAC initially disappears in the distal body’s lesser curvature and incisura, being these the most specific places to be analyzed. However, in chronic gastritis, when antral atrophy extends precisely through the incisura and lesser curvature of the distal body, there may be disappearance or deformity of the RAC, even in eradicated patients, making its analysis difficult. In these cases, we need to look for the RAC in the distal body mucosa away from atrophy.
Another important fact is that the last region where the RAC is preserved is in the proximal body and gastric fundus, places that are not recommended for evaluating bacterial infection.
3.2- With magnification
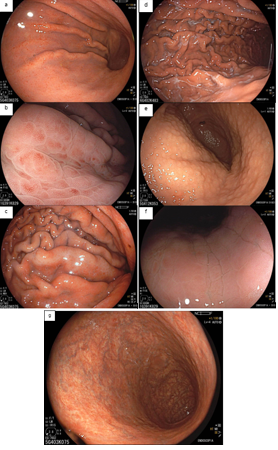
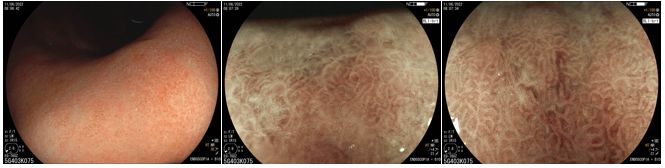
From a histomorphological perspective, with H. pylori infection, the crypts become larger and irregular, surrounded by erythema and grooves. We can no longer see the subepithelial capillary network, as inflammatory cells, edema, degenerated epithelium, and rupture of the microvascular network prevent its adequate visualization. The crypt orifices become asymmetrical and white due to the deposit of inflammatory content inside the glands. As atrophy expands, the marginal epithelium of the crypts becomes enlarged and has an irregular and elongated/curved shape. This is what we call “antralization” of the gastric body.
In a Brazilian study (Fiuza F, Martins BC, 2021), it was found that it is possible to identify these changes in the gastric mucosa (especially the presence or absence of RAC) using near focus technology, since gastroscopes with magnification are not yet widely available.
4. Findings of the treated stomach (post-eradication)
It still remains controversial whether H. pylori treatment can reverse atrophic gastritis and intestinal metaplasia. Moreover, it can take up to 10-15 years for the mucosa to recover and return to normal.
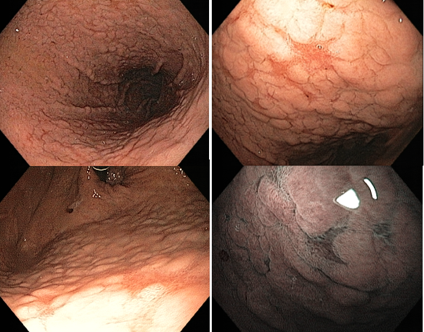
After eradication, the non-atrophic areas dissipate the inflammation and the atrophic areas become relatively reddish when compared to the adjacent mucosa. This confers the “map-like” pattern. This pattern may be associated with the development of both primary and metachronous gastric cancer even after effective treatment of H. pylori.
Another characteristic described is the “cracked” pattern, where grooves appear in the antral mucosa, indicating reparative mucosa.
Studies have shown that eradication therapy can alter the characteristics of the repaired stomach, causing difficulty in diagnosing early gastric cancer. That is why the post-eradication status must be distinguished from the negativity of H. pylori.
References
- Anagnostopoulos GK, Yao K, Kaye P, Fogden E, Fortun P, Shonde A, Foley S, Sunil S, Atherton JJ, Hawkey C, Ragunath K. High-resolution magnification endoscopy can reliably identify normal gastric mucosa, Helicobacter pylori-associated gastritis, and gastric atrophy. Endoscopy. 2007 Mar;39(3):202-7.
- Yao K. The endoscopic diagnosis of early gastric cancer. Ann Gastroenterol. 2013;26(1):11-22.
- Yagi K, Nakamura A, Sekine A. Comparison between magnifying endoscopy and histological, culture and urease test findings from the gastric mucosa of the corpus. Endoscopy. 2002 May;34(5):376-81.
- Yuan C, Lin XM, Ou Y, Cai L, Cheng Q, Zhou P, Liao J. Association between regular arrangement of collecting venules and Helicobacter pylori status in routine endoscopy. BMC Gastroenterol. 2021 Oct 20;21(1):389.
- Glover B, Teare J, Patel N. A systematic review of the role of non-magnified endoscopy for the assessment of H. pylori infection. Endosc Int Open. 2020 Feb;8(2):E105-E114.
- Qi Q, Guo C, Ji R, Li Z, Zuo X, Li Y. Diagnostic Performance of Magnifying Endoscopy for Helicobacter pylori Infection: A Meta-Analysis. PLoS One. 2016 Dec 19;11(12):e0168201.
- Weng CY, Xu JL, Sun SP, Wang KJ, Lv B. Helicobacter pylori eradication: Exploring its impacts on the gastric mucosa. World J Gastroenterol. 2021 Aug 21;27(31):5152-5170.
- Nishikawa Y, Ikeda Y, Murakami H, et al. Classification of atrophic mucosal patterns on Blue LASER Imaging for endoscopic diagnosis of Helicobacter pylori-related gastritis: A retrospective, observational study. PLoS One. 2018;13(3):e0193197.
- Toyoshima O, Nishizawa T, Koike K. Endoscopic Kyoto classification of Helicobacter pylori infection and gastric cancer risk diagnosis. World J Gastroenterol. 2020 Feb 7;26(5):466-477.
- Ono S, Dohi O, Yagi N, Sanomura Y, Tanaka S, Naito Y, Sakamoto N, Kato M. Accuracies of Endoscopic Diagnosis of Helicobacter pylori-Gastritis: Multicenter Prospective Study Using White Light Imaging and Linked Color Imaging. Digestion. 2020;101(5):624-630.
- Kato T, Yagi N, Kamada T, Shimbo T, Watanabe H, Ida K; Study Group for Establishing Endoscopic Diagnosis of Chronic Gastritis. Diagnosis of Helicobacter pylori infection in gastric mucosa by endoscopic features: a multicenter prospective study. Dig Endosc. 2013 Sep;25(5):508-18.
- Fiuza F, Maluf-Filho F, Ide E, Furuya CK Jr, Fylyk SN, Ruas JN, Stabach L, Araujo GA, Matuguma SE, Uemura RS, Sakai CM, Yamazaki K, Ueda SS, Sakai P, Martins BC. Association between mucosal surface pattern under near focus technology and Helicobacter pylori infection. World J Gastrointest Endosc. 2021 Oct 16;13(10):518-528.
How to cite this article
Nobre R, Baba E. ENDOSCOPIC FINDINGS RELATED TO H. PYLORI INFECTION. Endoscopy News; 2024, vol1. Available on: https://endoscopy.news/2024/01/27/endoscopic-findings-related-to-h-pylori-infection